Les Etats-Unis viennent d'autoriser la mise sur le marché d'un pilule connectée, capable de surveiller les patients.
(CCM) — La FDA américaine a récemment autorisé (lien en anglais) un premier médicament connecté, qui surveille la prise des traitements par les patients. Cette pilule connectée baptisée Abilify MyCite pourrait révolutionner le rapport que les malades entretiennent avec leur santé.
Une fois avalée, la pilule se loge dans l’estomac. De là, elle transmet ses données à un patch porté par le patient, qui à son tour peut communiquer avec un médecin ou un proche du malade. Pour fonctionner, le système repose sur un capteur mesurant en permanence l’acidité de l’estomac. Ainsi, il est possible de savoir si le malade prend régulièrement son traitement. La capteur permet également de suivre les doses de médicament ingérées, leur régularité et d'autres données de santé plus personnelles comme le sommeil, l'activité physique etc.
Cette pilule connectée pourrait donc permettre de surveiller très étroitement le comportement de patients à risque. Le mauvais suivi des traitements coûte cher au système de santé américain, et le micro-capteur mis au point par les chercheurs d’Abilify permettraient de le réduire considérablement.
Learn more / En savoir plus / Mehr erfahren:
https://www.scoop.it/t/21st-century-innovative-technologies-and-developments/?&tag=Research
https://www.scoop.it/t/21st-century-innovative-technologies-and-developments/?&tag=Medicine



 Your new post is loading...
Your new post is loading...





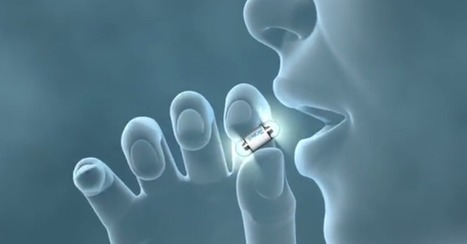






Les Etats-Unis viennent d'autoriser la mise sur le marché d'un pilule connectée, capable de surveiller les patients.
(CCM) — La FDA américaine a récemment autorisé (lien en anglais) un premier médicament connecté, qui surveille la prise des traitements par les patients. Cette pilule connectée baptisée Abilify MyCite pourrait révolutionner le rapport que les malades entretiennent avec leur santé.
Une fois avalée, la pilule se loge dans l’estomac. De là, elle transmet ses données à un patch porté par le patient, qui à son tour peut communiquer avec un médecin ou un proche du malade. Pour fonctionner, le système repose sur un capteur mesurant en permanence l’acidité de l’estomac. Ainsi, il est possible de savoir si le malade prend régulièrement son traitement. La capteur permet également de suivre les doses de médicament ingérées, leur régularité et d'autres données de santé plus personnelles comme le sommeil, l'activité physique etc.
Cette pilule connectée pourrait donc permettre de surveiller très étroitement le comportement de patients à risque. Le mauvais suivi des traitements coûte cher au système de santé américain, et le micro-capteur mis au point par les chercheurs d’Abilify permettraient de le réduire considérablement.
Learn more / En savoir plus / Mehr erfahren:
https://www.scoop.it/t/21st-century-innovative-technologies-and-developments/?&tag=Research
https://www.scoop.it/t/21st-century-innovative-technologies-and-developments/?&tag=Medicine