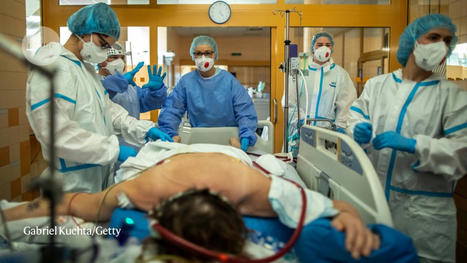
Evidence is growing that self-attacking ‘autoantibodies’ could be the key to understanding some of the worst cases of SARS-CoV-2 infection. More than a year after COVID-19 emerged, many mysteries persist about the disease: why do some people get so much sicker than others? Why does lung damage sometimes continue to worsen well after the body seems to have cleared the SARS-CoV-2 virus? And what is behind the extended, multi-organ illness that lasts for months in people with ‘long COVID’? A growing number of studies suggest that some of these questions might be explained by the immune system mistakenly turning against the body — a phenomenon known as autoimmunity. “This is a rapidly evolving area, but all the evidence is converging,” says Aaron Ring, an immunologist at the Yale School of Medicine in New Haven, Connecticut.
Early in the pandemic, researchers suggested that some people have an overactive immune response to COVID infection. Immune-system signalling proteins called cytokines can ramp up to dangerous levels, leading to ‘cytokine storms’ and damage to the body’s own cells. Clinical trials have now shown that some drugs that broadly dampen immune activity seem to reduce death rates in critically ill people, if administered at the right time. But scientists studying COVID are increasingly also highlighting the role of autoantibodies: rogue antibodies that attack either elements of the body’s immune defences or specific proteins in organs such as the heart. In contrast to cytokine storms, which tend to cause systemic, short-duration problems, autoantibodies are thought to result in targeted, longer-term damage, says immunologist Akiko Iwasaki, a colleague of Ring’s at Yale. Even healthy people make autoantibodies, but not generally in large amounts, and the molecules don’t usually seem to cause damage or attack the immune system. Yet researchers also have evidence that nefarious autoantibodies do have a role in many infectious diseases. There are several theories to explain how autoimmunity might emerge from COVID and other infections. Some people might be predisposed to producing autoantibodies that can then wreak havoc during an infection. Alternatively, infections could even trigger the production of autoantibodies. If researchers can establish the link, they might be able to come up with avenues for treatment, both for the repercussions of COVID and for other diseases caused by viruses.
Finding autoantibodies
In late September, a group led by Jean-Laurent Casanova at the Rockefeller University in New York City reported that more than 10% of 987 individuals with severe COVID-19 had antibodies that attacked and blocked the action of type 1 interferon molecules, which normally help to bolster the immune response against foreign pathogens1. That was a striking proportion, the researchers say, because people’s antibody repertoires are normally very dissimilar, and no one in a control group for the study had these antibodies. The researchers also saw the antibodies in people before their COVID-19 infection, so Casanova thinks that some people could be genetically predisposed to produce them. And the autoantibodies were more common in men than women — a possible factor in why COVID seems to hit men harder. The first evidence suggesting that autoantibodies against interferon might put people at higher risk of infectious disease was published in 1984, and evidence has accumulated since then, Casanova says. But now COVID is drawing more attention to the connection. “Now people understand the problem,” he says, “and all of a sudden they realize that what my lab has been doing for 25 years is actually pretty meaningful.” Casanova is now screening 40,000 people to see how many have pre-existing autoantibodies and determine whether their distribution by age, ancestry and gender matches that of severe COVID. Other research groups have supported Casanova’s autoantibody connection. Iwasaki, Ring and others screened 194 patients and hospital workers with varying severities of COVID for a wide range of autoantibodies. Their study, which was posted online in December and has not yet been peer reviewed, found a higher prevalence of autoantibodies against the immune system in infected individuals than in uninfected people. They found autoantibodies that attacked B cells, as well as some that attacked interferon.....
Published in Nature (Jan. 19, 2021):
https://doi.org/10.1038/d41586-021-00149-1
Via
Juan Lama

 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...