The U.S. Food and Drug Administration today approved Blincyto (blinatumomab) to treat patients with Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia (B-cell ALL), an uncommon form of ALL
Precursor B-cell ALL is a rapidly growing type of cancer in which the bone marrow makes too many B-cell lymphoblasts, an immature type of white blood cell. The Philadelphia chromosome is an abnormality that sometimes occurs in the bone marrow cells of leukemia patients. The National Cancer Institute estimates that 6,020 Americans will be diagnosed with ALL and 1,440 will die from the disease in 2014.
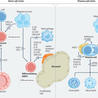
Blincyto is an example of immunotherapy, a treatment that uses certain parts of a person’s immune system to fight diseases such as cancer. Blincyto is the first approved drug that engages the body’s T-cells, a type of white blood cell or lymphocyte, to destroy leukemia cells. The drug acts as a connector between a protein called CD19, which is found on the surface of most B-cell lymphoblasts, and CD3, a protein on T-cell lymphocytes. It is intended for patients whose cancer returned after treatment (relapsed) or did not respond to previous treatment (refractory).
The safety and effectiveness of Blincyto were evaluated in a clinical study involving 185 adults with Philadelphia chromosome-negative relapsed or refractory precursor B-cell ALL. All participants were treated with Blincyto for at least four weeks via infusion, a method used to inject treatment into the bloodstream using a needle. Results showed 32 percent of participants had no evidence of disease (complete remission) for approximately 6.7 months.
Blincyto carries a boxed warning alerting patients and health care professionals that some clinical trial participants had problems with low blood pressure and difficulty breathing (cytokine release syndrome) at the start of the first treatment, experienced a short period of difficulty with thinking (encephalopathy) or other side effects in the nervous system. The most common side effects seen in Blincyto-treated participants were fever (pyrexia), headache, swelling of tissues (peripheral edema), fever with a low number of white blood cells (febrile neutropenia), nausea, low potassium (hypokalaemia), fatigue, constipation, diarrhea and tremor.
The FDA approved Blincyto with a Risk Evaluation and Mitigation Strategy (REMS), which consists of a communication plan to inform health care providers about the serious risks and the potential for preparation and administration errors.
.
Via
Krishan Maggon


 Your new post is loading...
Your new post is loading...







Editorial
How to train your T cell: genetically engineered chimeric antigen receptor T cells versus bispecific T-cell engagers to target CD19 in B acute lymphoblastic leukemia
Posted online on February 2, 2015. (doi:10.1517/14712598.2015.1009888)HTMLPDF (155 KB)PDF Plus (180 KB)ReprintsPermissionsMarco Ruella, and Saar Gill1University of Pennsylvania, Perelman School of Medicine, Translational Research Program, Philadelphia, PA, USA2University of Pennsylvania, Smilow Translational Research Center, Translational Research Program, Floor 8-196 C, 3400 Civic Center Boulevard, 19104-5157 Philadelphia, PA, USA +1 215 746 4880; marco.ruella@uphs.upenn.edu3University of Pennsylvania, Perelman School of Medicine, Hematology/Oncology, Philadelphia, PA, USA