 Your new post is loading...
 Your new post is loading...
Abstract This review of the July 2013 to December 2014 publications on cytokines and monoclonal antibodies covers bone morphogenic proteins, colony-stimulating factors, interferons, interleukins, tumor necrosis factor alfa, adalimumab, certolizumab, etanercept, golimumab, infliximab, abciximab, alemtuzumab, bevacizumab, cetuximab, daclizumab, natalizumab, ranibizumab, rituximab, tocilizumab and trastuzumab. KeywordsAdverse reactions; Cytokines; Monoclonal antibodies; Bone morphogenic proteins;Colony-stimulating factors; Interferons; Interleukins; Tumor necrosis factor alfa;Adalimumab; Certolizumab; Etanercept; Golimumab; Infliximab; Abciximab;Alemtuzumab; Bevacizumab; Cetuximab; Daclizumab; Natalizumab; Ranibizumab;Rituximab; Tocilizumab; Trastuzumab
Via Krishan Maggon
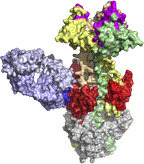
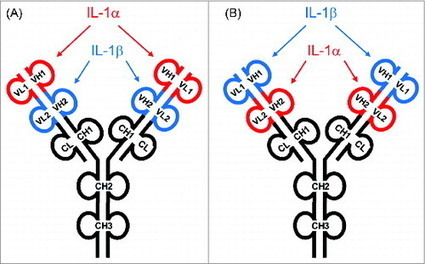
(2015). Generation and characterization of ABT-981, a dual variable domain immunoglobulin (DVD-IgTM) molecule that specifically and potently neutralizes both IL-1α and IL-1β. mAbs: Vol. 7, No. 3, pp. 605-619. doi: 10.1080/19420862.2015.1026501
Via Krishan Maggon
There are many reasons to be excited by the science being presented at this year’s American Society for Clinical Oncology meeting. The data...
Via Krishan Maggon
Highlights • Anti-CD20 mAbs combined with chemotherapy have become the standard treatment of Non-Hodgkin Lymphomas (NHL). • Mechanisms of action of this anti-CD20 mAbs in vivo are not fully understood. • Binding of rituximab to CD20 is not sufficient to heal every patient with a Non-Hodgkin Lymphoma. • We review the mechanisms of action and resistance of rituximab, and the efforts needed to overcome it. • We also focus on new drugs targeting pathways implicated in resistance to anti-CD20 mAbs. Abstract Anti-CD20 monoclonal antibodies (mAbs) have improved patient’s survival with non-Hodgkin lymphoma, when combined with chemotherapy. Several mechanisms of action have been reported, including antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity and induction of apoptosis. Despite the large amount of studies and published data, the role each mechanism played in vivo is not fully understood. Furthermore, the reason why a significant percentage of patients are refractory or resistant remains unknown. Several activated intracellular signaling pathways have been implicated in the mechanisms of resistance of rituximab. In the present manuscript, we review those mechanisms and new anti-CD20 mAbs, as well as the efforts being accomplished to overcome it, focusing on new drugs targeting pathways implicated in resistance to rituximab.
Via Krishan Maggon
Abstract With modern intensive combination polychemotherapy, the complete response (CR) rate in adults with acute lymphoblastic leukemia (ALL) is 80-90%, and the cure rate 40-50%. Hence there is a need to develop effective salvage therapies and combine novel agents with standard effective chemotherapy. ALL leukemic cells express several surface antigens amenable to target therapies, including CD20, CD22, and CD19. Monoclonal antibodies target these leukemic surface antigens selectively, and minimize off-target toxicity. When added to frontline chemotherapy, rituximab, an antibody directed against CD20, increases cure rates of adults with Burkitt leukemia from 40% to 80%, and those with pre-B ALL from 35% to 50%. Inotuzumab ozogamicin, a CD22 monoclonal antibody bound to calicheamicin, has resulted in marrow CR rates of 55% and a median survival of 6-7 months when given to patients with refractory-relapsed ALL. Blinatumomab, a biallelic T-cell engaging CD3-CD19 monoclonal antibody, also resulted in overall response rates of 40-50% and a median survival of 6.5 months in a similar refractory-relapsed population. Other promising monoclonal antibodies targeting CD20 (ofatumumab, obinutuzumab), or CD19 or CD20 and bound to different cytotoxins or immunotoxins are under development. Combined modalities of chemotherapy and the novel monoclonal antibodies are under investigation.
Via Krishan Maggon
Abstract Enhancing immune responses with immune-modulatory monoclonal antibodies directed to inhibitory immune receptors is a promising modality in cancer therapy. Clinical efficacy has been demonstrated with antibodies blocking inhibitory immune checkpoints such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) or PD-1/PD-L1. Treatment with ipilimumab, a fully human CTLA-4–specific mAb, showed durable clinical efficacy in metastatic melanoma; its mechanism of action is, however, only partially understood. This is a study of 29 patients with advanced cutaneous melanoma treated with ipilimumab. We analyzed peripheral blood mononuclear cells (PBMCs) and matched melanoma metastases from 15 patients responding and 14 not responding to ipilimumab by multicolor flow cytometry, antibody-dependent cell-mediated cytotoxicity (ADCC) assay, and immunohistochemistry. PBMCs and matched tumor biopsies were collected 24 h before (i.e., baseline) and up to 4 wk after ipilimumab. Our findings show, to our knowledge for the first time, that ipilimumab can engage ex vivo FcγRIIIA (CD16)-expressing, nonclassical monocytes resulting in ADCC-mediated lysis of regulatory T cells (Tregs). In contrast, classical CD14++CD16−monocytes are unable to do so. Moreover, we show that patients responding to ipilimumab display significantly higher baseline peripheral frequencies of nonclassical monocytes compared with nonresponder patients. In the tumor microenvironment, responders have higher CD68+/CD163+ macrophage ratios at baseline and show decreased Treg infiltration after treatment. Together, our results suggest that anti–CTLA-4 therapy may target Tregs in vivo. Larger translational studies are, however, warranted to substantiate this mechanism of action of ipilimumab in patients.
Via Krishan Maggon
PASADENA, Calif., April 20, 2015 /PRNewswire/ -- Meditope Biosciences, Inc., a biotechnology company developing novel antibody-based products using its proprietary technology, today announced presentation of data demonstrating the use of its SnAP technology platform for the development of antibody-drug conjugates (ADCs). The data were presented in an abstract at the AACR Annual Meeting 2015, taking place April 18-22 in Philadelphia. Scientists at the company demonstrated the successful transplantation of the meditope binding site to several antibodies. Meditope-peptides of different affinities were then selected by rational design and fine-tuned according to the requirements of specific application. For ADC development, data show that the meditope-peptides are conjugated with cytotoxic payloads and the higher the affinity of the meditope-peptide-drug conjugate for the antibody, the higher the complex's cytotoxic potential against antigen-positive tumor cells.
Via Krishan Maggon
Product and Process Innovation in the Development Cycle of Biopharmaceuticals #biologics #biologicals mAbs #biopharma
http://t.co/yV97kw0IAy Summary: Compared to small-molecule drugs, biopharmaceuticals require significantly more complex processes for R&D and production. In this work, we tracked and categorized patented biopharmaceutical technologies along the innovation cycles timeline using International Patent Classification (IPC) codes to show that the biopharmaceutical R&D process has been evolving from the conventional pattern of innovation. Our study builds on the latest research on innovation theories to provide empirical evidence that demonstrates reshaping of innovation processes through utilization of scientific knowledge. Based on our results, we provide strategic perspectives on the biopharmaceutical industry. Teaser: We tracked and categorized patented biopharmaceutical technologies along the innovation cycle timeline using IPC codes to show that the biopharmaceutical R&D process has been evolving from the conventional innovation pattern.
Via Krishan Maggon
Antibody Glycosylation and Its Impact on the PK and Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins http://t.co/7OLmZ4Sba2 Understanding the impact of glycosylation and keeping a close control on glycosylation of product candidates are required for both novel and biosimilar monoclonal antibodies (mAbs) and Fc-fusion protein development to ensure proper safety and efficacy profiles. Most therapeutic mAbs are of IgG class and contain a glycosylation site in the Fc region at amino acid position 297 and, in some cases, in the Fab region. For Fc-fusion proteins, glycosylation also frequently occurs in the fusion partners. Depending on the expression host, glycosylation patterns in mAb or Fc-fusions can be significantly different, thus significantly impacting the pharmacokinetics (PK) and pharmacodynamics (PD) of mAbs. Glycans that have a major impact on PK and PD of mAb or Fc-fusion proteins include mannose, sialic acids, fucose (Fuc), and galactose (Gal). Mannosylated glycans can impact the PK of the molecule, leading to reduced exposure and potentially lower efficacy. The level of sialic acid, N-acetylneuraminic acid (NANA), can also have a significant impact on the PK of Fc-fusion molecules. Core Fuc in the glycan structure reduces IgG antibody binding to IgG Fc receptor IIIa relative to IgG lacking Fuc, resulting in decreased antibody-dependent cell-mediated cytotoxicity (ADCC) activities. Glycoengineered Chinese hamster ovary (CHO) expression systems can produce afucosylated mAbs that have increased ADCC activities. Terminal Gal in a mAb is important in the complement-dependent cytotoxicity (CDC) in that lower levels of Gal reduce CDC activity. Glycans can also have impacts on the safety of mAb. mAbs produced in murine myeloma cells such as NS0 and SP2/0 contain glycans such as Galα1–3Galβ1–4N-acetylglucosamine-R and N-glycolylneuraminic acid (NGNA) that are not naturally present in humans and can be immunogenic when used as therapeutics. J Pharm Sci
Via Krishan Maggon
Abstract Antibody-drug conjugates (ADCs) are complex molecules composed of monoclonal antibodies conjugated to potent cytotoxic agents through chemical linkers. Because of this complexity, sponsors have used different approaches for the design of nonclinical studies to support the safety evaluation of ADCs and first-in-human (FIH) dose selection. We analyzed this data with the goal of describing the relationship between nonclinical study results and Phase 1 study outcomes. We summarized the following data from investigational new drug applications (INDs) for ADCs: plasma stability, animal study designs and toxicities, and algorithms used for FIH dose selection. Our review found that selecting a FIH dose that is 1/6th the highest non-severely toxic dose (HNSTD) in cynomolgus monkeys or 1/10th the STD10 in rodents scaled according to body surface area (BSA) generally resulted in the acceptable balance of safety and efficient dose-escalation in a Phase 1 trial. Other approaches may also be acceptable, e.g. 1/10th the HNSTD in monkeys using BSA or 1/10th the NOAEL in monkeys or rodents using body weight for scaling. While the animal data for the vc-MMAE platform yielded variable range of HNSTDs in cynomolgus monkeys, MTDs were in a narrow range in patients, suggesting that for ADCs sharing the same small molecule drug, linker and drug:antibody ratio, prior clinical data can inform the design of a Phase 1 clinical trial.
Via Krishan Maggon
Abstract Cytomegalovirus (CMV) is a virus that causes chronic infections in a large set of the population. It may cause severe disease in immunocompromised individuals, is linked to immunosenescence and implied to play an important role in the pathogenesis of cardiovascular diseases and cancer. Modulation of the immune system's abilities to manage the virus represent a highly viable therapeutic option and passive immunotherapy with polyclonal antibody preparations is already in clinical use. Defined monoclonal antibodies offer many advantages over polyclonal antibodies purified from serum. Human CMV-specific monoclonal antibodies have consequently been thoroughly investigated with respect to their potential in the treatment of diseases caused by CMV. Recent advances in human antibody technology have substantially expanded the breadth of antibodies for such applications. This review summarizes the fundamental basis for treating CMV disease by use of antibodies, the basic technologies to be used to develop such antibodies, and relevant human antibody specificities available to target this virus.
Via Krishan Maggon
Abstract Age-related macular degeneration (AMD) is the leading cause of vision loss in the western world. This multifactorial disease results from the combined contributions of age, environment and genetic predisposition. Antibody-based treatment of late-stage neovascular AMD with inhibitors of vascular endothelial growth factor has had great success, which is now the goal for currently untreatable AMD manifestations. The existence of an immune-privileged environment in the eye supports the feasibility of localized antibody therapy. Many different antibodies against various targets are being developed for the treatment of AMD, which reflects the etiological complexity of the disease. This review provides an overview of 19 potential therapeutic antibodies targeting angiogenesis, the complement system, inflammation or amyloid beta deposition in the eye. It summarizes the immunoglobulin structure, the specific target and study outcomes for each approach. The latter include beneficial results or adverse effects in AMD models and patients. Finally, this article discusses the challenges in the development of antibody-based drugs to treat degenerative processes in the posterior eye. In spite of these difficulties, to date, the following four antibodies have overcome the technical and preclinical hurdles and are being tested in active clinical studies: Lampalizumab, Sonepcizumab, GSK933776 and LFG316. We conclude that, while there are some antibody-based drugs that have made it into clinical practice, a successful transfer from bench to beside is still pending for many promising approaches.
Via Krishan Maggon
(2015). Macrophages are critical effectors of antibody therapies for cancer. mAbs: Vol. 7, No. 2, pp. 303-310.
Via Krishan Maggon
|
Clinical Development Strategies and Outcomes in First-in-Human Trials of Monoclonal Antibodies [Phase I and Clinic… http://t.co/JGqBGDq9rn Abstract Purpose We conducted a comprehensive review of the design, implementation, and outcome of first-in-human (FIH) trials of monoclonal antibodies (mAbs) to clearly determine early clinical development strategies for this class of compounds. Methods We performed a PubMed search using appropriate terms to identify reports of FIH trials of mAbs published in peer-reviewed journals between January 2000 and April 2013. Results A total of 82 publications describing FIH trials were selected for analysis. Only 27 articles (33%) reported the criteria used for selecting the starting dose (SD). Dose escalation was performed using rule-based methods in 66 trials (80%). The median number of planned dose levels was five (range, two to 13). The median of the ratio between the highest planned dose and the SD was 27 (range, two to 3,333). Although in 56 studies (68%) at least one grade 3 or 4 toxicity event was reported, no dose-limiting toxicity was observed in 47 trials (57%). The highest planned dose was reached in all trials, but the maximum-tolerated dose (MTD) was defined in only 13 studies (16%). The median of the ratio between MTD and SD was eight (range, four to 1,000). The recommended phase II dose was indicated in 34 studies (41%), but in 25 (73%) of these trials, this dose was chosen without considering toxicity as the main selection criterion. Conclusion This literature review highlights the broad design heterogeneity of FIH trials testing mAbs. Because of the limited observed toxicity, the MTD was infrequently reached, and therefore, the recommended phase II dose for subsequent clinical trials was only tentatively defined.
Via Krishan Maggon
As of today, there are a total of 367 monoclonal antibodies either on the market or in development. 124 are fully human, 146 are humanized, 37 are chimeric, and 56 are murine, or mice antibodies. Of these 367, 38 are FDA approved since the first got through in 1986. 16 of those 38 have been approved in the last 5 years alone, which translates to 42%. We are gaining traction quickly, it seems. Of the 38 approved mabs, 18 are humanized, 14 are fully human, 7 are chimeric, and only one is murine. The difference between the types is this: A chimeric mab is a cross between an animal and a human antibody engineered to react in a human setting. Humanized means that the antibody is mostly converted into a human form but originally came from an animal, usually a mouse. The term "fully human antibody" though is actually a bit misleading because in almost all cases it is not actually taken from a human. It is generally taken from a mechanism called a phage display, where human antibody genes are injected into a phage virus, which then expresses the antibodies on its coat. The best ones are then harvested from the virus. Think of it as microbiological agriculture. Zooming back out to mabs in general, the top 10 bestselling mabs in 2014 account for $68B of the $78B total mab market last year. Number 1 is the fully human adalimumab, or Humira for rheumatoid arthritis, which looks set to eventually overtake Lipitor as the biggest blockbuster drug of all time. Humira was discovered via phage display, along with (nearly) all other fully human mabs on the market or in development.
Via Krishan Maggon
(2015). An alternative assay to hydrophobic interaction chromatography for high-throughput characterization of monoclonal antibodies. mAbs: Vol. 7, No. 3, pp. 553-561.
Via Krishan Maggon
Highlights • First time demonstrating that CD47 blockade has anti-tumor effects against HCC. • In vitro CD47 blockade results in increase in phagocytosis of HCC cells. • In vivo CD47 blockade inhibits HCC tumor growth in hetero/orthotopic models. • CD47mAb400 is clearly more efficacious than widely-investigated B6H12 antibody. Abstract Human hepatocellular carcinoma (HCC) has a high rate of tumor recurrence and metastasis, resulting in shortened survival times. The efficacy of current systemic therapies for HCC is limited. In this study, we used xenograft tumor models to investigate the use of antibodies that block CD47 and inhibit HCC tumor growth. Immunostaining of tumor tissue and HCC cell lines demonstrated CD47 over-expression in HCC as compared to normal hepatocytes. Macrophage phagocytosis of HCC cells was increased after treatment with CD47 antibodies (CD47mAbs) that block CD47 binding to SIRPα. Further, CD47 blockade inhibited tumor growth in both heterotopic and orthotopic models of HCC, and promoted the migration of macrophages into the tumor mass. Our results demonstrate that targeting CD47 by specific antibodies has potential immunotherapeutic efficacy in human HCC.
Via Krishan Maggon
Abstract The convergence of advanced understanding of biology with chemistry has led to a resurgence in the development of antibody-drug conjugates (ADCs), especially with two recent product approvals. Design and development of ADCs requires the synergistic combination of the monoclonal antibody, the linker and the payload. Advances in antibody science has enabled identification and generation of high affinity, highly selective, humanized or human antibodies for a given target. Novel linker technologies have been synthesized and highly potent cytotoxic drug payloads have been created. As the first generation of ADCs utilizing lysine and cysteine chemistries moves through the clinic and into commercialization, second generation ADCs involving site specific conjugation technologies are being evaluated and tested. The latter aim to be better characterized and controlled, with wider therapeutic indices as well as improved pharmacokinetic-pharmacodynamic (PK-PD) profiles. ADCs offer some interesting physicochemical properties, due to conjugation itself, and to the (often) hydrophobic payloads that must be considered during their CMC development. New analytical methodologies are required for the ADCs, supplementing those used for the antibody itself. Regulatory filings will be a combination of small molecule and biologics. The regulators have put forth some broad principles but this landscape is still evolving.
Via Krishan Maggon
Pamlico Biopharma, Inc. was founded around human monoclonal antibody therapeutics and technologies from the OMRF and from Emory University. PAMLICO focuses on human monoclonal antibody therapeutics for human pathogens, cancer and autoimmune diseases. The lead project will address pneumonia caused by S pneumoniae. Pneumococcal pneumonia remains a major cause of morbidity and mortality worldwide. The magnitude of the clinical challenge is likely to increase as multiple strains of the bacteria have developed resistance to multiple antibiotics. PAMLICO’s lead project is a monoclonal antibody therapy for severe (PSI -V) community-acquired pneumonococcal pneumonia, which has a 20-35% mortality rate that has remained constant for 2 decades. The therapeutic goal is to reduce mortality by 20%, which the company anticipates demonstrating in a superiority study compared to standard care alone.
Via Krishan Maggon
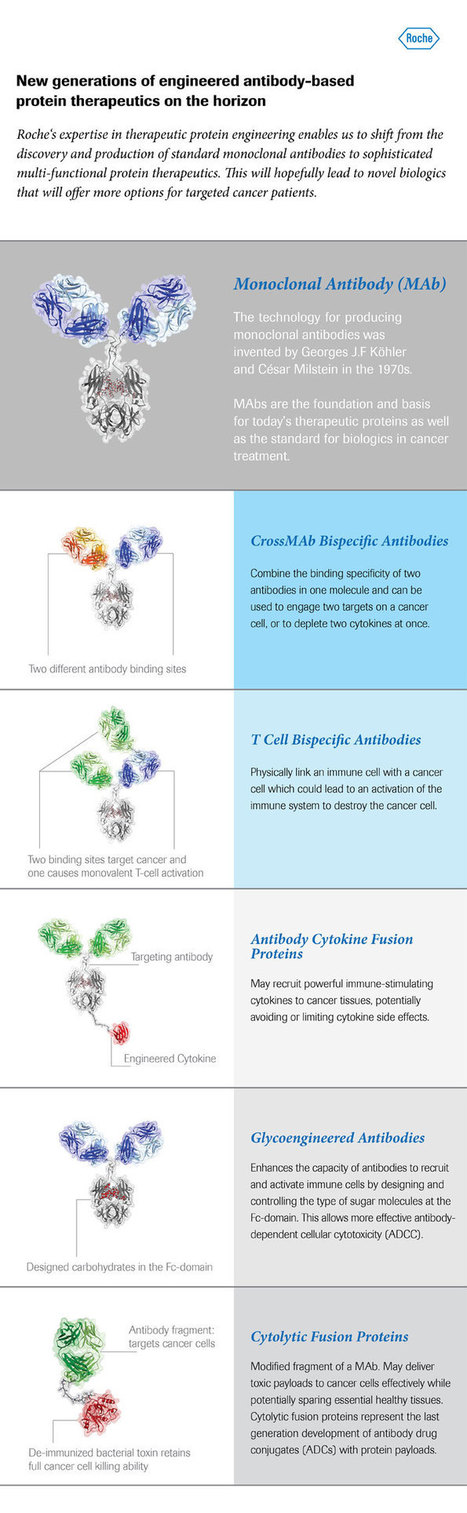
Roche currently has several different therapeutic antibody technologies in early clinical development which in the future could hopefully be a strong pillar in addressing the complexity of targeting more than 250 cancer diseases.
Via Krishan Maggon
NIST researchers have demonstrated the most precise method yet to measure the structural configuration of monoclonal antibodies, an important factor in determining the safety and efficacy of these biomolecules as medicines.
Via Krishan Maggon
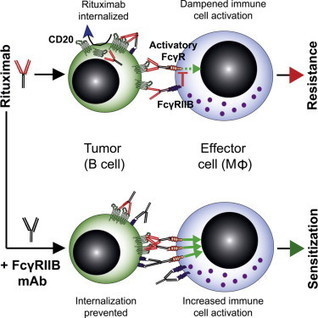
Highlights •Fully human hFcγRIIB (CD32B) antibodies overcome resistance to therapeutic antibodies•hFcγRIIB mAbs augment standard-of-care anti-CD20 therapy in vitro and in vivo•hFcγRIIB mAbs restore drug responsiveness in refractory CLL cells in vivo•hFcγRIIB mAbs help overcome cell- and niche-specific resistance mechanisms Summary Therapeutic antibodies have transformed cancer therapy, unlocking mechanisms of action by engaging the immune system. Unfortunately, cures rarely occur and patients display intrinsic or acquired resistance. Here, we demonstrate the therapeutic potential of targeting human (h) FcγRIIB (CD32B), a receptor implicated in immune cell desensitization and tumor cell resistance. FcγRIIB-blocking antibodies prevented internalization of the CD20-specific antibody rituximab, thereby maximizing cell surface accessibility and immune effector cell mediated antitumor activity. In hFcγRIIB-transgenic (Tg) mice, FcγRIIB-blocking antibodies effectively deleted target cells in combination with rituximab, and other therapeutic antibodies, from resistance-prone stromal compartments. Similar efficacy was seen in primary human tumor xenografts, including with cells from patients with relapsed/refractory disease. These data support the further development of hFcγRIIB antibodies for clinical assessment.
Via Krishan Maggon
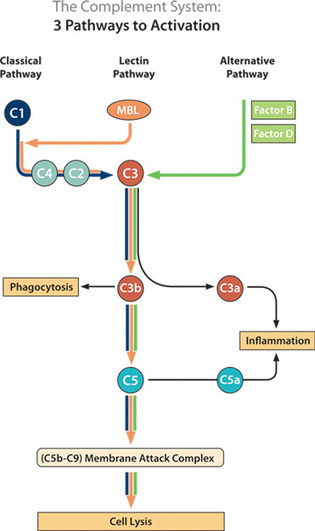
True North is developing novel humanized antibodies that inhibit pathogenic activity of the Complement pathway of the immune system. In contrast to conventional drug development approaches that focus primarily on inhibiting downstream components of the Complement cascade, True North’s therapeutics are designed to selectively target and block upstream processes in pathways of the Complement system. True North’s deepest drug discovery expertise involves the Classical Complement pathway, which is a pathway that has not previously been successfully pursued for drug development. True North’s lead drug candidate, TNT009, selectively targets the Classical Complement pathway to address fundamental disease mechanisms. Based on deep insights in Complement system biology, True North’s R&D engine is creating a pipeline of monoclonal antibodies focused on the treatment of Complement-mediated rare diseases in the hematologic, kidney transplant, and skin therapeutic areas.
Via Krishan Maggon
Highlights • 36 out of 39 approved monoclonal antibodies are native full-length IgG. • Antibody engineering may further increase potency and improve the safety profile of conventional antibodies. • Next generation of engineered antibodies may be even more efficient therapeutic molecules. • 18 multivalent engineered antibodies with promising preclinical results are now in clinical trials. • In December 2014, blinatumomab became the first bispecific antibody approved in US. The development of monoclonal antibody (mAb) technology has had a profound impact on medicine. The therapeutic use of first-generation mAb achieved considerable success in the treatment of major diseases, including cancer, inflammation, autoimmune, cardiovascular, and infectious diseases. Next-generation antibodies have been engineered to further increase potency, improve the safety profile and acquire non-natural properties, and constitute a thriving area of mAb research and development. Currently, a variety of alternative antibody formats with modified architectures have been generated and are moving fast into the clinic. In fact, the bispecific antibody blinatumomab was the first in its class to be approved by the US Food and Drug Administration (FDA) as recently as December 2014. Here, we outline the fundamental strategies used for designing the next generation of therapeutic antibodies, as well as the most relevant results obtained in preclinical studies and clinical trials.
Via Krishan Maggon
(2015). Molecular basis for antagonistic activity of anifrolumab, an anti-interferon–α receptor 1 antibody. mAbs: Vol. 7, No. 2, pp. 428-439.
Via Krishan Maggon
|



 Your new post is loading...
Your new post is loading...
















Available online 1 October 2015
In Press, Corrected Proof — Note to users