Engineered T cell CAR therapy
A revolution is taking place in the field of adoptive immunotherapy, where T cells armed with a Chimeric Antigen Receptor (CAR) are being used to fight cancerous cells. Genome engineering is at the heart of this revolution. This groundbreaking technology relies mainly on the use of engineered nucleases and makes it possible to modify the genome of the T cell to give it new properties. Specifically, the engineered T cell can be transformed into an allogeneic product, or it can resist existing cancer treatments or even overcome checkpoint inhibition. Genome engineering is regarded as one of the most important breakthroughs of recent years, and is about to revolutionize immunotherapy.
Decades of research have shown that it is possible to improve the ability of T cells to fight diseases by genome engineering. For example, T cells can be engineered by adding a new gene (called a chimeric antigen receptor or CAR) that will boost their ability to recognize and destroy cancer cells. Another possibility for T cells is to inactivate an existing gene that damages the immune response.
The possibilities provided by T cell genome engineering are endless. Very sophisticated strategies can be designed at will to open the door for a new era of treatments in indications such as infectious diseases, autoimmune diseases, and cancer.
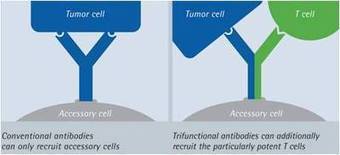
Chimeric Antigen Receptors (CARs) are artificial molecules that, when present at the surface of immune effector cells, will enable them to recognize a desired protein (antigen) and trigger the killing of cells harboring this antigen at their surface (target cells).
These receptors are becoming one of the most promising approaches to fight cancer, through the development of adoptive cell transfer therapies. Indeed, immune cells (most usually T-lymphocytes) can be engineered to express a CAR able to recognize proteins present at the surface of cancer cells. Upon cell-to-cell contact between effector and targeted cells, antigen recognition will activate the effectors, giving them the signal to attack their targets, and leading ultimately to the killing of cancer cells.
CARs are constructed by assembling domains from different proteins, each of which enables the chimeric molecule to carry out specific functions. The most common CAR architecture comprises an extracellular domain containing a region that recognizes the targeted antigen and a spacer region that links it to the transmembrane domain (the part of the protein that spans the cellular membrane). This is then followed by an intracellular domain, responsible for transmitting an activation signal to the cell upon antigen recognition, causing the CAR-engineered cell to attack the tumor cell.
The target-binding moiety is usually derived from an antibody, while the intracellular portion can include, besides the domain leading to cell activation and cytotoxic response, one or more domains from co-stimulatory receptor proteins that could enhance the proliferative capacity and survival of the “therapeutic” cells.
Cellectis is currently developing a collection of CARs targeting antigens present on cells from various types of cancer, as well as a proprietary multi-chain architecture of these artificial receptors, aiming to further increase the efficacy of adoptive cell therapies in the future.
CELLECTIS’ UCART19 RECEIVES ADVANCED-THERAPY MEDICINAL PRODUCT CLASSIFICATION FROM EMAJune 23, 2014
READ MORE READ THE PRESS RELEASEPFIZER AND CELLECTIS ENTER INTO GLOBAL STRATEGIC CANCER IMMUNOTHERAPY COLLABORATIONJune 18, 2014
Via
Krishan Maggon



 Your new post is loading...
Your new post is loading...












Highlights
•
Melanoma treatment has been transformed using T-cell checkpoint antibodies.
•
Antibodies to CTLA-4 and PD-1 have had the largest impact on melanoma management.
•
Combination T-cell checkpoint therapy holds great promise for clinical development.
•
OX40 and 4-1BB are T cell costimulators with clinical potential in melanoma.