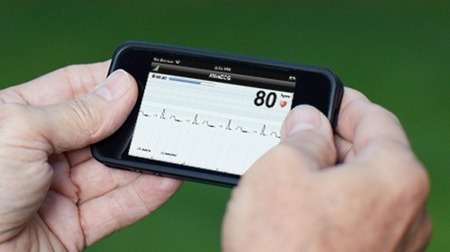
AliveCor’s smartphone Heart Monitor has received FDA approval and will go on sale to healthcare professionals in the United States in January 2013. The AliveCor Heart Monitor allows the recording, display, storing, transferring, and evaluation of single-channel electrocardiogram (ECG) rhythms using an iPhone 4 or 4S.
The Class II medical device consists of a self-powered case that attaches to the back of an iPhone, which is running the associated heart monitor app. The phone and case communicate with one another wirelessly, though the phone doesn't need to be paired to the device.



 Your new post is loading...
Your new post is loading...