Your new post is loading...
 Your new post is loading...
Torres A, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. View this article via: CrossRef PubMed Google Scholar Gao CA, et al. Gearing up for battle: harnessing adaptive T cell immunity against gram-negative pneumonia. Front Cell Infect Microbiol. 2022;12:934671.
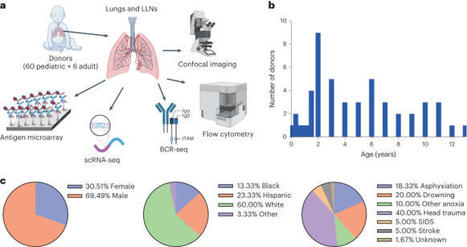
Infants and young children are more susceptible to common respiratory pathogens than adults but can fare better against novel pathogens like severe acute respiratory syndrome coronavirus 2. The mechanisms by which infants and young children mount effective immune responses to respiratory pathogens are unknown. Through investigation of lungs and lung-associated lymph nodes from infant and pediatric organ donors aged 0–13 years, we show that bronchus-associated lymphoid tissue (BALT), containing B cell follicles, CD4+ T cells and functionally active germinal centers, develop during infancy. BALT structures are prevalent around lung airways during the first 3 years of life, and their numbers decline through childhood coincident with the accumulation of memory T cells. Single-cell profiling and repertoire analysis reveals that early life lung B cells undergo differentiation, somatic hypermutation and immunoglobulin class switching and exhibit a more activated profile than lymph node B cells. Moreover, B cells in the lung and lung-associated lymph nodes generate biased antibody responses to multiple respiratory pathogens compared to circulating antibodies, which are mostly specific for vaccine antigens in the early years of life. Together, our findings provide evidence for BALT as an early life adaptation for mobilizing localized immune protection to the diverse respiratory challenges during this formative life stage. Young children frequently encounter respiratory pathogens that elicit immune responses in developing lungs. Farber and colleagues examine rare lung tissue samples obtained from pediatric organ donors and find age-dependent formation of bronchus-associated lymphoid tissue (BALT), which peaks at 3 years of age and dissipates thereafter. Profiling of BALT lymphocytes indicates that repertoire and functional differences exist between the lung, draining lymph nodes and circulating cells.
An integrated cell atlas of the lung in health and disease - Nature Medicine
Abstract B cells are critical mediators of humoral immune responses in the airways through antibody production, antigen presentation, and cytokine secretion. In addition, a subset of B cells, known...
Legionella-Infected Macrophages Engage the Alveolar Epithelium to Metabolically Reprogram Myeloid Cells and Promote Antibacterial Inflammation
Even after thousands of years, tuberculosis remains a killer – but AI-assisted research into diverse populations may help
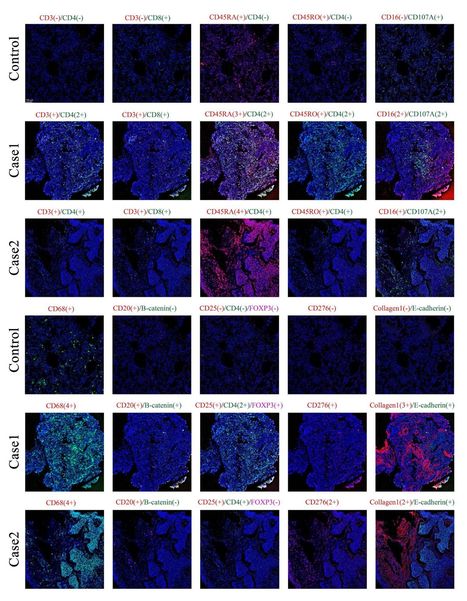
Background: A previous report of lung biopsies from a patient with coronavirus disease 2019 (COVID-19) and acute respiratory distress syndrome (ARDS) identified mononuclear cell infiltration but not the type of mononuclear cells ( 1 ).
Chronic obstructive pulmonary disease (COPD) is characterized by continuous flow limitation and the immune system including macrophages and regulatory T lymphocytes (Tregs) is involved in COPD pathogenesis.
Respiratory syncytial virus–bronchiolitis is a major independent risk factor for subsequent asthma, but the causal mechanisms remain obscure. We identified that transient plasmacytoid dendritic cell (pDC) depletion during primary Pneumovirus infection alone predisposed to severe bronchiolitis in early life and subsequent asthma in later life after reinfection. pDC depletion ablated interferon production and increased viral load; however, the heightened immunopathology and susceptibility to subsequent asthma stemmed from a failure to expand functional neuropilin-1+ regulatory T (T reg) cells in the absence of pDC-derived semaphorin 4a (Sema4a). In adult mice, pDC depletion predisposed to severe bronchiolitis only after antibiotic treatment. Consistent with a protective role for the microbiome, treatment of pDC-depleted neonates with the microbial-derived metabolite propionate promoted Sema4a-dependent T reg cell expansion, ameliorating both diseases. In children with viral bronchiolitis, nasal propionate levels were decreased and correlated with an IL-6high/IL-10low microenvironment. We highlight a common but age-related Sema4a-mediated pathway by which pDCs and microbial colonization induce T reg cell expansion to protect against severe bronchiolitis and subsequent asthma.
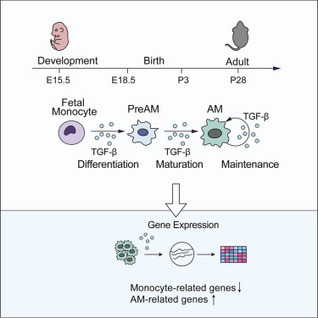
Highlights • TGF-β is essential for the differentiation and homeostasis of AMs • The genesis of other macrophages does not require TGF-β receptor signaling • TGF-β controls AMs in an autocrine manner • TGF-β regulates expression of genes associated with AM differentiation and fate Summary Alveolar...
|
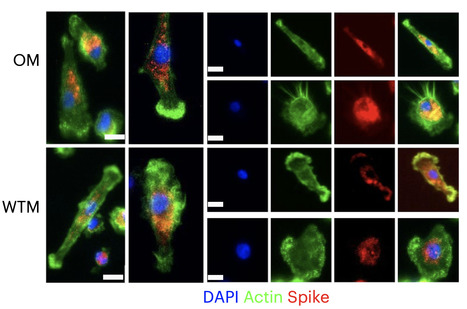
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA generally becomes undetectable in upper airways after a few days or weeks postinfection. Here we used a model of viral infection in macaques to address whether SARS-CoV-2 persists in the body and which mechanisms regulate its persistence. Replication-competent virus was detected in bronchioalveolar lavage (BAL) macrophages beyond 6 months postinfection. Viral propagation in BAL macrophages occurred from cell to cell and was inhibited by interferon-γ (IFN-γ). IFN-γ production was strongest in BAL NKG2r+CD8+ T cells and NKG2Alo natural killer (NK) cells and was further increased in NKG2Alo NK cells after spike protein stimulation. However, IFN-γ production was impaired in NK cells from macaques with persisting virus. Moreover, IFN-γ also enhanced the expression of major histocompatibility complex (MHC)-E on BAL macrophages, possibly inhibiting NK cell-mediated killing. Macaques with less persisting virus mounted adaptive NK cells that escaped the MHC-E-dependent inhibition. Our findings reveal an interplay between NK cells and macrophages that regulated SARS-CoV-2 persistence in macrophages and was mediated by IFN-γ. Huot et al. show that interferon-γ (IFN-γ) regulates the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in bronchoalveolar macrophages from cynomolgus macaques up to 18 months postinfection. Published in Nat. Immunology (Nov. 2, 2023): https://doi.org/10.1038/s41590-023-01661-4
Via Juan Lama
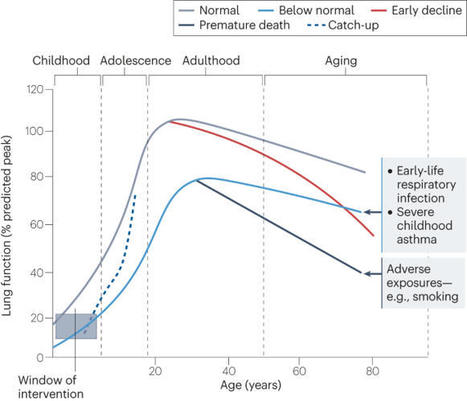
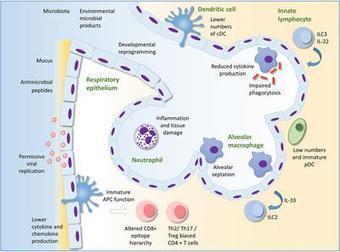
Respiratory infections are common in infants and young children. However, the immune system develops and matures as the child grows, thus the effects of infection during this time of dynamic change may have long-term consequences. The infant immune system develops in conjunction with the seeding of the microbiome at the respiratory mucosal surface, at a time that the lungs themselves are maturing. We are now recognizing that any disturbance of this developmental trajectory can have implications for lifelong lung health. Here, we outline our current understanding of the molecular mechanisms underlying relationships between immune and structural cells in the lung with the local microorganisms. We highlight the importance of gaining greater clarity as to what constitutes a healthy respiratory ecosystem and how environmental exposures influencing this network will aid efforts to mitigate harmful effects and restore lung immune health. Lloyd and Saglani review the immunology of early-life respiratory infections and how developmental immunity determines lifelong lung health.
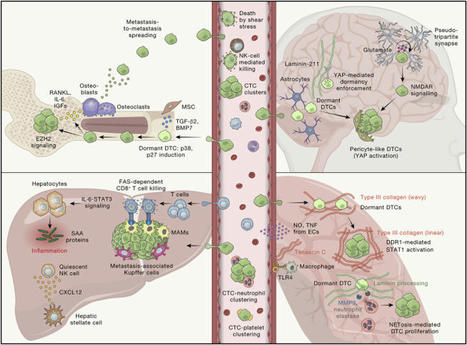
de Visser and Joyce review the complex interplay between the tumor and its microenvironment
throughout cancer evolution and discuss the tumor cell-intrinsic, cell-extrinsic,
and systemic factors that may be exploited for rational design of anti-cancer treatments.
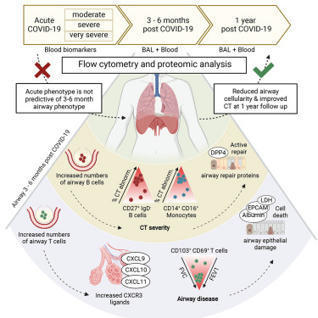
Many individuals recovering from acute SARS-CoV-2 infection suffer prolonged respiratory
dysfunction for months to years after viral clearance. Vijayakumar, Boustani, Ogger,
Papadaki et al. show that individuals with persistent symptoms 3-6 months after infection
have an altered airway immune cell landscape and evidence of ongoing lung damage.
Importantly, different immune cell types correlate with the severity of distinct aspects
of ongoing respiratory disease.
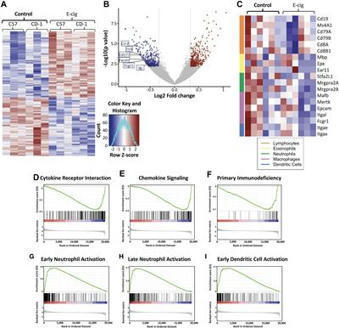
Conventional smoking is known to both increase susceptibility to infection and drive inflammation within the lungs. Recently, smokers have been found to be at higher risk of developing severe forms of coronavirus disease 2019 (COVID-19).
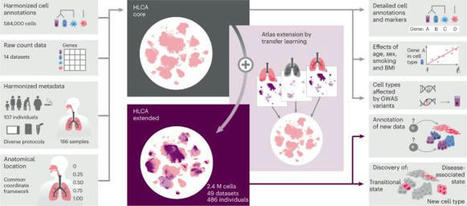
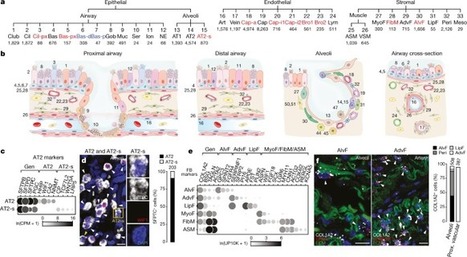
Although single-cell RNA sequencing studies have begun to provide compendia of cell expression profiles1–9, it has been difficult to systematically identify and localize all molecular cell types in individual organs to create a full molecular cell atlas. Here, using droplet- and plate-based single-cell RNA sequencing of approximately 75,000 human cells across all lung tissue compartments and circulating blood, combined with a multi-pronged cell annotation approach, we create an extensive cell atlas of the human lung. We define the gene expression profiles and anatomical locations of 58 cell populations in the human lung, including 41 out of 45 previously known cell types and 14 previously unknown ones. This comprehensive molecular atlas identifies the biochemical functions of lung cells and the transcription factors and markers for making and monitoring them; defines the cell targets of circulating hormones and predicts local signalling interactions and immune cell homing; and identifies cell types that are directly affected by lung disease genes and respiratory viruses. By comparing human and mouse data, we identified 17 molecular cell types that have been gained or lost during lung evolution and others with substantially altered expression profiles, revealing extensive plasticity of cell types and cell-type-specific gene expression during organ evolution including expression switches between cell types. This atlas provides the molecular foundation for investigating how lung cell identities, functions and interactions are achieved in development and tissue engineering and altered in disease and evolution. Expression profiling on 75,000 single cells creates a comprehensive cell atlas of the human lung that includes 41 out of 45 previously known cell types and 14 new ones.
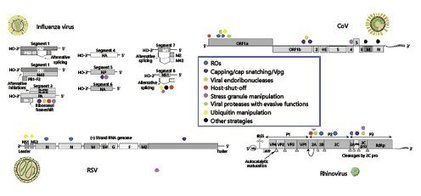
The impact of respiratory virus infections on the health of children and adults can be very significant.Yet, in contrast to most other childhood infections as well as other viral and bacterial diseas...
Credit: Toshio Hirano and Masaaki Murakami. Immunity.April 22, 2020 Research into how the SARS-CoV-2 virus induces death is suggesting...
medRxiv - The Preprint Server for Health Sciences
![Figure][1]
Ectopic lymphoid structures form in a wide range of inflammatory conditions, including infection, autoimmune disease, and cancer. In the context of infection, this response can be beneficial for the host: influenza A virus infection–induced pulmonary ectopic germinal centers give rise to more broadly cross-reactive antibody responses, thereby generating cross-strain protection. However, despite the ubiquity of ectopic lymphoid structures and their role in both health and disease, little is known about the mechanisms by which inflammation is able to convert a peripheral tissue into one that resembles a secondary lymphoid organ. Here, we show that type I IFN produced after viral infection can induce CXCL13 expression in a phenotypically distinct population of lung fibroblasts, driving CXCR5-dependent recruitment of B cells and initiating ectopic germinal center formation. This identifies type I IFN as a novel inducer of CXCL13, which, in combination with other stimuli, can promote lung remodeling, converting a nonlymphoid tissue into one permissive to functional tertiary lymphoid structure formation.
[1]: pending:yes
Early life is a period of particular susceptibility to respiratory infections and symptoms are frequently more severe in infants than in adults. The neonatal immune system is generally held to be deficient in most compartments; responses to innate stimuli are weak, antigen presenting cells have poor immunostimulatory activity and adaptive lymphocyte responses are limited, leading to poor immune memory and ineffective vaccine responses. For mucosal surfaces such as the lung, which is continuously exposed to airborne antigen and to potential pathogenic invasion, the ability to discriminate between harmless and potentially dangerous antigens is essential, to prevent inflammation that could lead to loss of gaseous exchange and damage to the developing lung tissue. We have only recently begun to define the differences in respiratory immunity in early life and its environmental and developmental influences. The innate immune system may be of relatively greater importance than the adaptive immune system in the neonatal and infant period than later in life, as it does not require specific antigenic experience. A better understanding of what constitutes protective innate immunity in the respiratory tract in this age group and the factors that influence its development should allow us to predict why certain infants are vulnerable to severe respiratory infections, design treatments to accelerate the development of protective immunity and design age specific adjuvants to better boost immunity to infection in the lung.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...