 Your new post is loading...
 Your new post is loading...
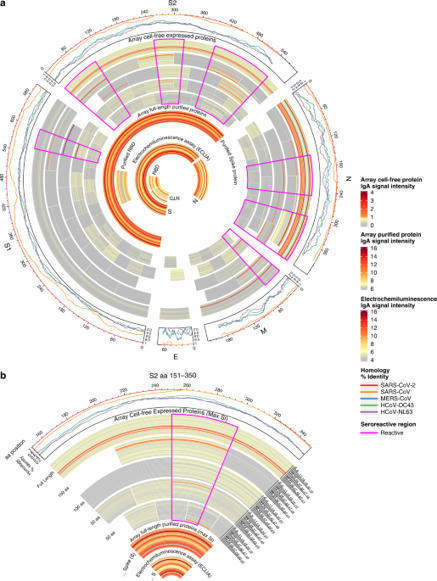
One potential mechanism for protection from SARS-CoV-2 in children is through passive immunity via breast milk from a mother infected with the novel coronavirus. The primary objectives of this study were to establish the presence of SARS-CoV-2-specific IgA and IgG and to characterize the antigenic regions of SARS-CoV-2 proteins that were reactive with antibodies in breast milk. Between March 2020 and September 2020, 21 women with confirmed SARS-CoV-2 infection were enrolled in Mommy’s Milk. Participants donated serial breast milk samples around their time of illness. Breast milk samples were used to probe a multi-coronavirus protein microarray containing full-length and variable-length overlapping fragments of SARS-CoV-2 proteins. Samples were also tested against S and N proteins by electrochemiluminescence assay. The breast milk samples contained IgA reactive with a variety of SARS-CoV-2 antigens. The most IgA-reactive SARS-CoV-2 proteins were N (42.9% of women responded to ≥1 N fragment) and S proteins (23.9% responded to ≥1 fragment of S1 or S2). IgG responses were similar. A striking observation was the dissimilarity between mothers in antibody recognition, giving distinct antibody reactivity and kinetic profiles. Individual COVID-19 cases had diverse and unique milk IgA profiles following the onset of symptoms.
ROR-γt+ regulatory T cells (Tregs) of the colon can prevent excessive inflammation but also delay pathogen clearance. How these cells are regulated ha…
To determine the transfer of rituximab, an anti-CD20 monoclonal antibody widely used for neurologic conditions, into mature breast milk.Breast milk samples were collected from 9 women with MS who received rituximab 500 or 1,000 mg intravenous once or ...
San Diego researchers are taking a close look into why break milk is so nutritious—and investigating how to make those nutrients stretch further
In the first study of its kind, LSU Health New Orleans researchers report that women's pre-pregnancy overweight or obesity produces changes in breast milk, which can affect infant growth.
The image shared on Twitter surprised and shocked many people.
A large-scale analysis in humans reveals that indirect breastfeeding using pumped milk is associated with the depletion of oral bacteria and a higher abundance of potential pathogens compared with direct breastfeeding at ...
J Transl Med. 2019 Jan 3;17(1):3. doi: 10.1186/s12967-018-1760-8.Review...

|
Suggested by
LIGHTING
|
Human Milk Oligosaccharides Exhibit Antimicrobial and Antibiofilm Properties against Group B Streptococcus
Breast milk bacteria influence the establishment and development of the infant gut microbiome with continued impact after solid food introduction.
Brandtzaeg, P., 1974: Human secretory component. II. Physicochemical characterization of free secretory component purified from colostrum
|
The importance of breastfeeding in low-income and middle-income countries is well recognised, but less consensus exists about its importance in high-income countries.
In low-income and middle-income countries, only 37% of children younger than 6 months of age are exclusively breastfed.
In a recent PLoSONE study, researchers found that maternal cytotoxic T cells transferred via breast milk to suckling pups home to Peyer’s patches in the pups, and are much more active than the newborns’ cytotoxic T cells.
Via Krishan Maggon
Glycerol monolaurate (GML), a compound found in human breast milk, fights against the effects of harmful bacteria while allowing beneficial bacteria to thrive. GML also inhibits inflammation in epithelial cells, helping to prevent both bacterial and viral infections of the gut.
Abstract Breast milk is not only a completely adapted nutrition source for the newborn but also an impressive array of immune‐active molecules that afford protection against infections and shape mucosal immune responses. Decisive imprinting events might be modulated during the first months of life with potential health long‐term effects, enhancing the importance of breastfeeding as a major influence on the immune system correct development and modifying disease susceptibility. The aim of this review was to clarify the link between breastfeeding and autoimmune diseases, inquiring the related mechanisms, based on data available in the literature. Being breastfed was associated with a lower incidence of diabetes, celiac disease, multiple sclerosis and asthma, explained by the protection against early infections, anti‐inflammatory properties, antigen‐specific tolerance induction, and regulation of infant's microbiome. The protective role of human milk in idiopathic juvenile arthritis, rheumatoid arthritis, and inflammatory bowel diseases remains controversial. On the other hand, the breastfeeding mother faces a health‐challenging period in life. High levels of prolactin may lead either to the development of autoimmune diseases in susceptible mothers or exacerbations of current immune‐mediated disorders. These features raise the question if mothers with autoimmune diseases, mainly systemic lupus erythematosus, should avoid breastfeeding. 1 INTRODUCTION Breast milk is the result of 200 million years of Darwinian pressure on mammalian lactation as the source of infant's nourishment.1 Bo Vahlquist (1909‐1978), a renowned pediatrician once said “In all mammalian species the reproductive cycle comprises both pregnancy and breast‐feeding: in the absence of latter, none of these species, man included, could have survived”.2 Diet is one of the most important environmental exposures in early life.3 Currently, the World Health Organization recommends that infants should be exclusively breastfed up to 6 months of age and continue with a partial pattern until the infant is at least 2 years old.4 Breastfeeding has multifactorial determinants and needs supportive measures on many levels, which can represent a barrier for women who attempt it5; still, many are unaware of its great importance on growth and development.6 In 1932, Grulee et al7 analyzed 20 000 healthy infants during the first year of life, relating breastfeeding with a lower incidence of morbidity and mortality. Previously, in the nineteenth century, antibodies were the first immunological molecules discovered in breast milk, boosting the possibility that human milk could have an influence on the development of children immune system.8 Indeed, human milk supplies an impressive array of immune‐active molecules, metabolites, oligosaccharides, microbial content, vitamins, and other nutrients that provide protection against infections and modulates mucosal immune responses.4, 9 At this time, more than 80 different autoimmune diseases have been described in which the leading role is characterized by an inappropriate immune reaction.10 The possibility that decisive imprinting events might be modulated during the first months of life, with potential long‐term effects, sparks the importance of breastfeeding in the correct development of the immune system, altering disease susceptibility, mainly in immune‐mediated conditions.11, 12 Breast milk is not only a completely adapted nutrition source but also a personalized medicine for the infants, programming to some degree their future health.13 For breastfeeding mothers, this period may represent a great health challenge. As prolactin plays an important role on immune modulation,10 physiologic hyperprolactinemia related to continuous breast stimulation may be involved either on the onset of autoimmune diseases or on the exacerbation of current ones.14 2 HUMAN MILK AMONG THE STAGES OF LACTATION Milk is a complex fluid, the composition of which gradually changes according to the stage of lactation, infant feeding, health status (mother and newborn), maternal diet, environment, and genetics.15 Colostrum is the first milk produced and is characterized for containing over 250 potentially immunologically active proteins, including enzymes (lactoferrin, lysozyme, etc.), hormones, immunoglobulins, cytokines, signaling molecules, soluble receptors and inflammatory mediators.8, 16 The high content of bioactive proteins evidenced that colostrum's main role is immunological rather than nutritional.17 Antimicrobial factors present in human milk possess resistance to degradation by the newborn's digestive enzymes, protecting the mucosal surfaces and eliminating pathogens without initiating inflammatory reactions, while the immune system matures.18 Furthermore, the abundance and diversity of indigestible oligosaccharides promote the optimal microbiota colonization which will condition future immune homeostasis.19 A few days after delivery, tight junctions of the mammary epithelium start to close, the rate of sodium/potassium declines, and the lactose concentration rises, indicating the secretory activation and production of transitional milk. Two weeks postpartum, the milk is considered fully mature.20 It is characterized by decreasing relative concentrations of the immunologically active molecules, while the volume and nutritional requirements increase to fulfill the growing infant needs.21 3 BREAST MILK AND IMMUNE SYSTEM MODULATION The postnatal period is crucial for the newborn's immune system maturation. This process is characterized by the development of a balance between Th1/Th2 response.22 The thymus is the central immune system organ where bone marrow‐derived precursors undergo differentiation and selection before they are apt for migration to the periphery23; therefore, thymus plays a central role in the establishment of T‐cell‐mediated immunity.23, 24 Hasselbalch et al25 reported that the thymus is considerably larger in breastfed infants when compared to formula‐fed. Subsequently, Jeppese et al26 not only corroborated the previous findings but also discovered a correlation between breastfeeding and CD8+ T cells. The mechanism by which breastfeeding may influence thymus size is unclear, although pathways involving interleukin 7 (IL‐7) are considered to play a critical role in thymopoiesis, and Tgd lymphocytes output from the intestinal mucosa.27, 28 In addition, breast milk is known to modulate infant's intestinal microbiome,29 which is the main source of bacterial metabolites (Figure 1). This has been proved to play a leading role in T‐cell development and differentiation and may be the missing link between the benefits of breastfeeding in the intestinal microbiome and enhanced thymus size in breastfed infants.30, 31 3.1 Immunological components of human milk Human milk supplies an impressive array of immunologically active molecules (Table 1) involved in complex interactions and influencing infant's outcomes.9, 32 This effect is due to the amazing nature of the interaction between mother and baby. Breast milk will not only nourish the newborn, but also provide a complete arsenal of immune components that will contribute as protective factors and as development and maturation factors (Table 1). The protective factors are known to contribute against infections by providing direct immunity (passive), and immune regulatory effects based on anti‐inflammatory capabilities. They ensure the adequate environment in the infant's gastrointestinal tract by preventing microbiome dysbiosis, therefore preserving the immune barrier functions of the epithelium.33, 34 The newborn immune system can be looked at as a very complex machine with amazing capabilities, yet it does not know how to use it. The development and maturation factors will promote, as their name implies, the maturation, differentiation and development of the local immune system, known as the gut‐associated lymphoid tissue (GALT), and the systemic immune system. Furthermore, it will also promote the development of immune tolerance.22, 35 Components Role Function P D/M Proteins Lactoferrin ● Antimicrobial, iron carrier Lactadherin, lysozyme, defensins ● Antimicrobial MicroRNAs ● ● Lymphocyte development, inflammatory mediator Cells Lymphocytes ● ● (T cells > 80%); lymphocyte development, inflammatory mediator Macrophages ● ● Phagocytosis, pathogen defense, T‐cell stimulation Neutrophils ● Unknown, may play a role in maternal protection Breast milk stem cells ● Secretion of growth factors; tissue homeostasis, repair, and/or regeneration Immunoglobulins sIgA/IgA ● ● Antimicrobial, pathogen binding inhibition IgM/IgG ● ● Antimicrobial, antibody‐mediated cytotoxicity Cytokines IL‐1b ● ● Inflammatory mediator, intestinal and immune system trophic factor IL‐2 ● ● Modulate T‐cell development IL‐6 ● ● Intestinal trophic factor, inflammatory mediator, B‐cell activation IL‐8 ● ● Intestinal trophic factor, recruitment of maternal leukocytes IL‐10 ● ● Intestinal trophic factor, anti‐inflammatory cytokine, tolerance promoter IFN‐g ● ● Stimulate Th1 inflammatory response, suppress Th2 allergic response TNF‐a ● ● Proinflammatory Chemokines and other soluble factors CXCR‐1/CXCR‐2 ● ● Cytokine receptor CXCL‐9 (MIP) ● ● Antimicrobial, NK and T‐cell chemoattractant sCD14 ● Inflammatory mediator, promotor of lymphocyte differentiation and activation IP‐10 ● Antimicrobial, NK and T‐cell chemoattractant MCP‐1 ● ● T‐cell chemoattractant TNF‐RI ● Reduce TNF‐a activity, mediate inflammation G‐CSF ● Stimulate neutrophil growth and differentiation, intestinal trophic factor Growth factors TGF‐b, TGF‐a, EGF ● ● Intestinal trophic factor, promote tolerance IGF‐1 and IGF‐2 ● Development and maturation of gastrointestinal cells Oligosaccharides HMO's ● ● Promote colonization of commensal bacteria, antimicrobial effects (pathogen binding, change gut′s pH) Gangliosides ● ● Prebiotic, suppress inflammation Other immunological compounds LCPUFAs ● ● Promote tolerance, Th1 and Th2 response Nucleotides ● Intestinal trophic factor, promote differentiation and activation of lymphocytes and macrophage, NK activity Osteoprotegerin ● ● May regulate Th1/Th2 balance Food antigens ● Promote tolerance EGF, epidermal growth factor; G‐CSF, granulocyte‐macrophage colony‐stimulating factor; HMO, human milk oligosaccharides; IFN‐g, interferon gamma; Ig, immunoglobulin; IGF, insulin‐like growth factor; IL, interleukin; IP, interferon gamma (IFN)‐g‐inducible protein; LCPUFA, long‐chain polyunsaturated fatty acid; MCP, monocyte chemotactic protein 1; MIP, monokine induced by IFN‐g; TGF, transforming growth factor; TNF‐a, tumor necrosis factor‐alpha. Recent studies show that milk composition may contain different compounds according to the newborn's condition, suggesting a permanent dynamic interaction between mother and baby. Riskin et al36 found an increased number of leukocytes in breast milk when the infant has an infection. Additionally, there are significant differences between immunological factors for term and preterm breast milk.17, 37 In the last few years, analytical advancements have allowed a better characterization of the human milk composition, discovering new structures like stem cells, microbiome elements, miRNA, increasing our knowledge every day.38, 39 3.2 Breast milk and microbiome Breastfeeding influences the gut microbiome and metagenome. Recent studies revealed that the main imprinting events on the newborn immune system might be mediated through the microbiome, highlighting the early influence of the infant's diet nature.6, 40 Its capability to regulate hosts responses depends on the bacterial composition of milk. Abnormal colonization has a long‐term effect on the immune system, mainly with respect to metabolic and autoimmune diseases.41 The microbiome of breastfed infants has copious amounts of bifidobacteria and Lactobacillus species, promoted by the rich bioactive factors present in the human milk, like oligosaccharides(HMOs) that function as natural prebiotics.42 Therefore, there is indeed specificity between the infant's microbiome and breastfeeding, resulting in different bacterial‐induced effects on metabolism and immunity. Curiously, this interaction is based on the mother's enteromammary axis, with the release of dendritic cells containing live maternal gut microbiome (which select non‐pathogenic bacteria from the gut lumen and carry them to the lactating mammary gland), T cells expressing gut‐derived β7 integrins, and plasma cells producing specific immunoglobulin into the milk.43, 44 Naturally, the cytokines present in breast milk also depend on the mother's immunological experience and this heritage will be transmitted to the infant's immature immune system, encouraging tolerogenic responses.45 Studies have reported similar microbiome profiles in human milk, independently of newborns time of gestation or delivery mode.46 Bergstom et al47 reported that the major factor affecting infant microbiome up to 12 months of age was the cessation of breastfeeding. 3.3 Breast milk and immune tolerance A healthy mucosal immune system is capable of distinguishing nutritional antigens and non‐harmful microbes from pathogens, promoting adequate immunological reactions by suppression of cellular and humoral immune responses to ingested antigens. This “controlled responses” are called “oral tolerance” and are influenced by the intestinal microbiota.48 Diseases due to a defect in immune tolerance, such as celiac disease or type 1 diabetes are less common in children who were breastfed, suggesting a protective effect.49 Recent data reported that breastfeed‐induced protection may be explained by early antigen‐specific tolerance induction, both by the transfer of antigens through milk and the presence of factors that affect the newborn's immune maturation and responses,50 adjusting for the deficiency of Immunoglobulin (Ig) synthesis during the first year of life. Indeed, studies have exposed the presence of antigens involved in the pathogenesis of multiple immune‐mediated diseases, such as soluble maternal semi‐allogenic HLA molecules,51 maternal cells, gliadin, ovalbumin, intact human insulin, and food allergens, such as peanut and bovine beta‐lactoglobulin.52 Each mother will produce different milk in accordance with her previous antigen exposure,53 as well as immune responses to transferred antigens, permeability of the mammary gland epithelium,54 microbiome‐influencing factors, gut growth factors in milk, and the presence of tolerogenic molecules.11, 55 4 BREASTFEEDING AND AUTOIMMUNE DISEASES 4.1 Breastfeeding and diabetes 4.1.1 Type 1 diabetes Type 1 diabetes (T1D) is known to be an immune‐mediated disease causing destruction of pancreatic B cells eventually leading to complete and lifelong dependence on exogenous insulin.56 Although genetic susceptibility variants play a crucial role in the development of T1D, increased incidence over the past 50 years strongly suggests an important role for non‐genetic factors.57, 58 The hypothesis that breastfeeding could protect against type 1 diabetes was proposed more than 30 years ago by Borch‐Johnsen et al,59 and since then, several mechanisms have been implied. The reason why human milk might protect against T1D remains uncertain, although numerous theories have been considered for the protective effect of breastfeeding, such as lower incidence of potentially diabetogenic infections, later exposure to dietary antigens, healthier gut microbiota, and correct maturation of the newborn's gut.6, 60, 61 Several positive health outcomes have been associated with breastfeeding4, 62 so far. Cardwell et al63 reported that being breastfed for more than 3 months or exclusively breastfed for more than 2 weeks after birth was associated with 15%‐30% lower risk of T1D in childhood. This association was also found in genetically high‐risk birth controls,64 although other studies were not able to find such affiliation.65, 66 Recently, Lund‐Blix et al performed a large cohort study involving 155 392 children to clarify the relation between the duration of full or any breastfeeding and risk of type 1 diabetes. The authors concluded that children who were never breastfed had a twofold increased risk for developing type 1 diabetes. In the same cohort, authors found no association between the age of introduction of specific solid foods (vegetables, fruits, and berries), or breastfeeding while introducing solid foods, and T1D. This result might be due to cultural differences in infant feeding practices worldwide.64, 66, 67 In contrast, the Diabetes and68 Autoimmunity Study in the Young (DAISY) suggested that the introduction of any solid food before 4 months and after 6 months of age predicted the development of T1D. Likewise, Virtanen et al69 concluded from the Type 1 Diabetes Prediction and Prevention Project (DIPP) study a close link between the risk of islet autoimmunity and early introduction of root vegetables. A recent field of investigation relates microbiome and immune‐mediated diseases. Following birth, early gut microbiota undergoes several changes triggered by the environment. As dietary changes strongly shift the microbial community composition early in life, breastfeeding could play a main role preventing dysbiosis. Interestingly, recent data suggest that alterations in the proportions of short‐chain fatty acids (SCFAs), such as butyrate, might be a potential mechanism contributing to the risk of developing T1D.70, 71 Ongoing large prospective birth control studies, such as DAISY, TEDDY (The Environmental Determinants of Diabetes in the Young), DIPP, as well as the first nutritional primary prevention study of type 1 diabetes, Trial to Reduce IDDM in the Genetically at Risk (TRIGR), will help to clarify the influence of nutrition in the pathogenesis of T1D. 4.1.2 Type 2 diabetes Type 2 diabetes (T2D) is an endocrine pathological state affecting young individuals and adults, which has seen a significant increase in the last 30 years, particularly in children worldwide.72 The etiology is highly heterogeneous, including genetic and environmental factors. These environmental factors, such as diet, may be altered to prevent the onset and the development of the disease.72, 73 The absence of breastfeeding or its improper pattern is recognized as a prime contributive factor in the future development of obesity and metabolic syndrome. Indeed, previous studies related proper breastfeeding with a 24% decreased risk of T2D in childhood.74 According to earlier data, a systematic review directly correlated the duration of breastfeeding and the risk of development of childhood T2D and obesity in neonates.75 More recently, Halipchuk et al76 performed a large retrospective case‐control study to identify the factors associated with the development of childhood‐onset T2D and suggested that exclusive breastfeeding decreases the risk of development of childhood‐onset type 2 diabetes. Unless preventive and intervening efforts are successful, the T2D pandemic and its complications will continue to have an intergenerational effect, affecting many more individuals worldwide.77 4.2 Breastfeeding and idiopathic juvenile arthritis Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease of childhood.78 The pathogenesis and etiology of JIA are unclear, although interactions among genetic factors, immune mechanisms, and environmental exposures are thought to contribute in most cases. Studies relating breastfeeding and JIA have been controversial. Mason et al79 suggested that breastfeeding may protect against JIA, although subsequent studies did not support this theory.80, 81 A prospective cohort study reported that a longer duration of breastfeeding (both total and exclusive) may protect against development of JIA,82 finding an increased risk of JIA in children who were breastfed for less than 4 months. Recently, a cohort study associated breastfeeding with an earlier but milder presentation of JIA and with different patterns of arthritis. Authors justified the earlier onset with the differences in ILAR (International League of Associations for Rheumatology) categories observed between the groups, with a higher proportion of PsA/ERA (Psoriatic Arthritis/Enthesitis‐related arthritis) in the never‐breastfed group, which tend to present at a later age.83 The reason why human milk can influence the susceptibility to certain subtypes of JIA is unknown, although studies have related it with differences in microbiome between children with and without enthesitis‐related arthritis.83, 84 Indeed, breastfeeding could be indirectly linked to JIA by the gut microbiome, as altered microbial profiles have been identified among JIA patients.84 However, further studies are needed to clarify the role of breastfeeding in JIA disease risk.85 4.3 Breastfeeding and rheumatoid arthritis Rheumatoid arthritis (RA) is the most common autoimmune inflammatory joint disease worldwide, characterized by a distinctive pattern of bone and joint destruction.86 Several studies have investigated the influence of breastfeeding and the risk of developing RA, but the results were inconsistent.87-89 An investigation performed by Colebatch reported that in HLA‐DR4‐negative children, rheumatoid factor‐positive infants were less likely to have been breastfed for >3 months when compared with rheumatoid factor‐negative children, although this effect was not seen in HLA‐DR4‐positive infants.90 A recent meta‐analysis by Chen et al91 included 1672 patients with RA and aimed to estimate the association between breastfeeding and RA risk, and an inverse association was found in the overall study population, relating a decreased risk of RA no matter if breastfeeding was longer or shorter than 12 months. 4.4 Breastfeeding and multiple sclerosis Multiple sclerosis (MS) is the most common immune‐mediated disease of the central nervous system affecting young adults.92 Evidence suggests that environmental factors act long before MS becomes symptomatic, suggesting the existence of a prodromal phase of this disease. Conradi et al93 recently associated breastfeeding for at least 4 months as a protective factor against the risk of developing MS; however, other studies still debate the impact of breastfeeding duration in determining the risk for MS.94 Applying data from a large multinational case‐control study (EnvIMS), Ragnedda et al95 described an increased risk of MS in males who were breastfed for less than 4 months. The protective role of human milk in the pathogenesis of immune‐mediated MS could be related with its capacity of promoting the immune system development, safeguarding from toxic agents and pathogens, and modulation of immune responses,96, 97 including immunomodulatory effects by interleukin (IL)‐10 production and the anti‐inflammatory properties of transforming growth factor (TGF)‐β.98 4.5 Breastfeeding and celiac disease Celiac disease (CD) is a systemic immune‐mediated disorder caused by the ingestion of gluten‐containing grains in genetically susceptible individuals.99 Genetic background plays a crucial role in the predisposition to celiac disease,100 although better long‐term health was observed in CD patients who were breastfed.101 This fact may be due to the delayed introduction of gluten in daily diet and expanding the latency time between the introduction of gluten and the development of the disease,102 lower incidence of gastrointestinal infections in the newborn who is breastfed,103 and the prevention of gut dysbiosis.104 Meta‐analysis and systemic review, including all studies between 1966 and 2004, found that infants who were breastfed had a 52% risk reduction in developing CD when compared with those who were not breastfed at the time of gluten introduction.105 Hanson et al106 reported that CD was prevented when gluten was introduced in small doses during breastfeeding. More recently, a meta‐analysis proved that the risk of CD was significantly decreased in infants who were breastfed at the time of gluten introduction.105, 107 Actually, being breastfed at the time of gluten introduction was related with a latter onset of CD.100, 105, 107 Interestingly, intestinal dysbiosis may promote an abnormal response to gluten in predisposed individuals,108 a fact that could be due to the role of specific bacteria in the modulation of infant's immune responses.109 However, it is still not clear whether human milk truly provides permanent tolerance acquisition or just a symptomatic reduction and delayed diagnosis of CD.110 Future studies should investigate the effect of different intervention strategies in CD, including nutrition, immunomodulation, and the role of the microbiome.111 4.6 Breastfeeding and inflammatory bowel diseases Inflammatory bowel diseases (IBD) are known as a chronic inflammatory condition of the gastrointestinal tract, manifesting has 2 major disorders: ulcerative colitis and Crohn disease.112 The exact pathogenesis of IBD remains unknown, and part of the underlying mechanism is believed to be a deregulated host immune response to intestinal flora, in genetically susceptible individuals.12, 113 The major risk for developing IBD is having a family history of the disease.113 The greatest incidence of IBD is in early adulthood, bolstering the fact that early exposures might force future susceptibility. Human milk compounds not only protect against infections but also influence the immune tolerance and bacterial colonization of the infant's gut.114 Considerable investigations found that breastfeeding was protective for IBD,115-117 mainly on early onsets.118 However, other studies failed to relate breastfeeding with IBD.119, 120 This result heterogeneity might be explained by differences in definition (exclusive or nonexclusive) and duration of breastfeeding. 4.7 Breastfeeding and asthma The allergic response to common environmental allergens has been regarded as an important mechanism in the development of airway inflammation in patients with asthma.121 Antibodies directed toward β‐adrenergic receptors were found in sera of some patients with asthma supporting an autoimmune role in the impairment of β‐adrenergic responsiveness.122 Until now, many studies suggested that prolonged and exclusive breastfeeding reduces the risk of wheezing and asthma in infancy and early childhood. Human milk contains allergens, allergen‐specific immunoglobulins, immune complexes, and immunosuppressive cytokines that are transferred through the milk and might modify the immune response to allergens and decrease susceptibility to immune‐mediated disorders.123 A questionnaire‐based cross‐sectional study performed in 2016 by Huang et al concluded that exclusive breastfeeding for 3‐6 months and either partial or exclusive breastfeeding for more than 6 months were associated with significantly reduced risk of asthma. The infant gut microbiome is emerging as an important hypothesis; accordingly, Johnson et al124 recently related the abnormal gut microbiota with the risk of developing asthma and allergic diseases, suggesting the protective role of human milk in maintaining a healthy gut microbial environment. 4.8 Breastfeeding and other autoimmune diseases In 1990, Fort attempted to assess the prevalence of breastfeeding in patients with autoimmune thyroid disease (ATD), which may have a protective role in the development of this disease. Results were unable to document any relationship between the history and duration of breastfeeding and development of ATD; however, a higher prevalence of feedings with soy‐containing formulas in early infancy was found.125 Recently, Simard et al126 evaluated the link between breastfeeding and systemic lupus erythematosus (SLE) among 2 prospective cohort studies, the Nurses’ Health Study and the Nurses’ Health Study II, but found no statistically significant association between duration and SLE incidence. Further research in this field may lead to novel links and strategies of breastfeeding and immune disorders. To summarize and for better comprehension, the relation between breastfeeding and autoimmune diseases is illustrated in Figure 2. 5 BREASTFEEDING MOTHER, PROLACTIN, AND AUTOIMMUNE DISEASES A high prevalence of autoimmune diseases among females suggests a crucial role for gender and hormonal involvement in the pathogenesis of these diseases. The association between prolactin and the immune system has been extensively investigated in the last 2 decades. Prolactin is a polypeptide hormone and member of the immunoneuroendocrine network. It is synthesized not only in the hypophysis but also in other several sites such as neurons, mammary epithelium, skin, prostate, immune organs, and cells. Prolactin secreted by the hypophysis acts as a hormone while the one produced in other organs functions as a cytokine.127 Even though the main physiological role of prolactin is to regulate the mammary gland and ovaries, sustain lactation, and control maternal behavior, currently the evidence points to a correlation between prolactin and autoimmune diseases (Table 2) specially, systemic lupus erythematosus (SLE).128 Organ‐specific diseases Systemic diseases Diabetes mellitus type 1 Celiac disease Addison′s disease Grave′s disease Hashimoto′s thyroiditis Multiple sclerosis Uveitis Lymphocytic hypophysitis Rejection of heart transplant Systemic lupus erythematosus Systemic sclerosis Sjögren syndrome Rheumatoid arthritis Reactive arthritis 5.1 Prolactin and immune modulation Genes which encode prolactin are located in the short arm of chromosome 6, near to HLA‐DRB1. Polymorphisms of the human prolactin gene might have implications in the pathogenesis of autoimmune diseases.127, 129 Studies have demonstrated that hyperprolactinemia impacts B‐cell tolerance via several pathways: (1) decreasing apoptosis in transitional B cells, (2) impairing B‐cell clonal deletion, (3) deregulating receptor editing, and finally (4) decreasing activation of anergic B cells.130-132 Besides, prolactin has proved to induce the expression of IL‐2 receptor and production of interferon‐γ and IL‐1.133 It regulates the maturation of dendritic cells and thymocytes, leading to an increased interferon‐α production and an enhancement of pro‐B‐cell generation.134, 135 5.2 Prolactin and systemic lupus erythematosus Systemic lupus erythematosus is more common in women of reproductive age. Hyperprolactinemia has been described in multiple autoimmune diseases and has shown an intimate correlation with SLE.136 Recently, Song et al137 performed a meta‐analysis to evaluate the relationship between circulating prolactin level and SLE. The author succeeded to demonstrate a significantly positive correlation between prolactin levels and disease activity. A large cohort study performed by Orbach et al138 suggests a role for hyperprolactinemia in lupus and its manifestations, including serositis and anemia. In addition, bromocriptine therapy proved to be beneficial in decreasing disease activity and improving activity scores.139, 140 The discontinuation of therapy was associated with lupus flares.141, 142 Taken together, these findings raise the question whether mothers with SLE should breastfeed their infants. Current evidence points specially to the benefits of treatment with bromocriptine in non‐responsive lupus cases or in flare prevention in postpartum.10, 143 6 CONCLUSIONS The benefits of breastfeeding are well recognized worldwide. Current scientific evidence supports the beneficial role of breastfeeding in several immune‐mediated diseases, although a few exact pathways are yet to be elucidated. Recent technological progresses allowed a better characterization of the human milk composition, opening new horizons to our knowledge for a better understanding of its capabilities, and plausible mechanisms which play a protective role in a heterogeneous pool of diseases with health long‐term effects. On the other hand, the benefits of breastfeeding for mothers with autoimmune diseases are debatable. The role of prolactin in the modulation of the immune system is undeniable, and recent data showed a positive correlation between hyperprolactinemia and lupus manifestations, disease activity, and relapses. Current evidence proved that treatment with bromocriptine is beneficial to non‐responsive lupus cases or on flare prevention in the postpartum. 7 KEY NOTES According to early breastfeeding patterns, decisive imprinting events on the immune system might be modulated during the first months of life, with potential long‐term health effects. Human milk benefits may be explained by the protection against early infections, anti‐inflammatory properties, early antigen‐specific tolerance induction, and regulation of infant's microbiome. Breastfeeding was associated with a protective role in T1D and CD. Benefits in T2D were related to the prevention of obesity and metabolic syndrome. In AS, breastfeeding showed benefit by promoting healthy microbiota, which is suspected to be influenced by HLA‐B27. Infants who were breastfed for at least 4 months show a decreased incidence of MS, through the immunomodulatory effects of IL‐10 production and the anti‐inflammatory properties of TGF‐β. Breastfeeding was associated with lower incidences of asthma during childhood, mainly because of a shaped immune response to allergens. Association between breastfeeding and JIA, RA, and IBD was controversial. A positive correlation between hyperprolactinemia and SLE manifestations, disease activity, and relapses was found. Evidence proved the benefits of treatment with bromocriptine on non‐responsive lupus cases and on flare prevention in the postpartum. ACKNOWLEDGMENTS The authors would like to express sincere gratitude to Professor Eitan Israeli for his priceless contribution to this article. CONFLICT OF INTEREST The authors have no potential conflict of interests in authorship or publication. REFERENCES Citing Literature
It's well-known that breastfed babies tend to be healthier than babies given a bottle filled with formula, especially in their first year of life, and the benefits are numerous. What's more, the longer a baby is breastfed, the more far-reaching...
The soon-to-be trend is being driven by a boom in studies showing human milk oligosaccharide, a property in breast milk, boosts good bacteria in the gut.
Front Immunol. 2019 Jan 22;10:16. doi: 10.3389/fimmu.2019.00016. eCollection 2019.Review...
J Crohns Colitis. 2018 Nov 12. doi: 10.1093/ecco-jcc/jjy186.[Epub ahead of print]...
In this issue Ohsaki et al. (<https://doi.org/10.1084/jem.20171163>;) explain how breastfeeding can prevent the onset of food allergies in offspring by instructing T reg formation via neonatal Fc receptor (FcRn)–mediated transfer and uptake of allergen-containing IgG immune complexes (Ig-ICs) by gut dendritic cells (DCs).
Human milk provides a very wide range of nutrients and bioactive components, including immune factors, human milk oligosaccharides, and a commensal microbiota. These factors are essential for interconnected processes including immunity programming and the development of a normal infant gastrointestinal microbiome. Newborn immune protection mostly relies on maternal immune factors provided through milk. However, studies dealing with an in-depth profiling of the different immune compounds present in human milk and with the assessment of their natural variation in healthy women from different populations are scarce. In this context, the objective of this work was the detection and quantification of a wide array of immune compounds, including innate immunity factors (IL1β, IL6, IL12, INFγ, TNFα), acquired immunity factors (IL2, IL4, IL10, IL13, IL17), chemokines (IL8, Groα, MCP1, MIP1β), growth factors (IL5, IL7,EGF, GCSF, GMCSF,TGFβ2) and immunoglobulins (IgA, IgG, IgM), in milk produced by healthy women of different ethnicities living in different geographic, dietary, socio-economic, and environmental settings. Among the analyzed factors, IgA, IgG, IgM, EGF, TGFβ2, IL7, IL8, Groα, MIP1β were detected in all or most of the samples collected in each population and, therefore, this specific set of compounds might be considered as the “core” soluble immune factors in milk produced by healthy women worldwide. This approach may help define which immune factors are (or are not) common in milk produced by women living in various conditions, and to identify host, lifestyle, and environmental factors that affect the immunological composition of this complex biological fluid.
New Abbott data presented this week, at the 50th Annual Congress of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) in Prague, further supports the important role Human Milk Oligosaccharides (or HMOs) play in supporting the immune system of formula-fed babies. It all starts in the digestive system.
|
 Your new post is loading...
Your new post is loading...
 Your new post is loading...
Your new post is loading...


























Diversity of repertoires!