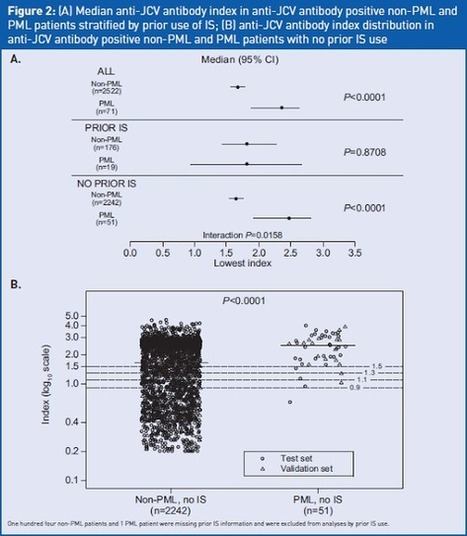
Your new post is loading...
 Your new post is loading...
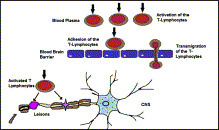
AbstractOBJECTIVE: Novel treatments such as natalizumab and fingolimod achieve their therapeutic efficacy in multiple sclerosis (MS) by blocking access of subsets of immune cells into the central nervous system, thus creating nonphysiological intrathecal immunity. In contrast, daclizumab, a humanized monoclonal antibody against the alpha chain of the IL-2 receptor, has a unique mechanism of action with multiple direct effects on innate immunity. As cellular intrathecal abnormalities corresponding to MS have been well defined, we asked how daclizumab therapy affects these immunological hallmarks of the MS disease process. METHODS: Nineteen subpopulations of immune cells were assessed in a blinded fashion in the blood and 50-fold concentrated cerebrospinal fluid (CSF) cell pellet in 32 patients with untreated relapsing-remitting MS (RRMS), 22 daclizumab-treated RRMS patients, and 11 healthy donors (HDs) using 12-color flow cytometry. RESULTS: Long-term daclizumab therapy normalized all immunophenotyping abnormalities differentiating untreated RRMS patients from HDs. Specifically, strong enrichment of adaptive immune cells (CD4+ and CD8+ T cells and B cells) in the CSF was reversed. Similarly, daclizumab controlled MS-related increases in the innate lymphoid cells (ILCs) and lymphoid tissue inducer cells in the blood and CSF, and reverted the diminished proportion of intrathecal monocytes. The only marker that distinguished daclizumab-treated MS patients from HDs was the expansion of immunoregulatory CD56(bright) NK cells. INTERPRETATION: Normalization of immunological abnormalities associated with MS by long-term daclizumab therapy suggests that this drug's effects on ILCs, NK cells, and dendritic cell-mediated antigen presentation to CD4+ and CD8+ T cells are critical in regulating the MS disease process.
Via Krishan Maggon
The purpose of this study was to identify the molecular target of the human monoclonal antibody HIgM12. HIgM12 reverses motor deficits in chronically demyelinated mice, a model of MS. Here we identified polysialic acid (PSA) attached to the Neural Cell Adhesion Molecule (NCAM) as the antigen for HIgM12 by using different NCAM knockout strains and through PSA removal from the NCAM protein core. Antibody binding to CNS tissue and primary cells, antibody-mediated cell adhesion and neurite outgrowth on HIgM12-coated nitrocellulose was detected only in the presence of PSA as assessed by Western blotting, immunoprecipitation, immunocytochemistry and histochemistry. We conclude that HIgM12 mediates it's in vivo and in vitro effects through binding to PSA and has the potential to be an effective therapy for MS and neurodegenerative diseases
Via Krishan Maggon
A critical appraisal of daclizumab use as emerging therapy in multiple sclerosis http://t.co/iyRnRnfa4A Introduction: Daclizumab (DAC) is a mAb that binds to CD25, a receptor on the surface of lymphocytes for IL-2, a chemical messenger in the immune system. This prevents activation and proliferation of lymphocytes, which are involved in the immune attack in multiple sclerosis (MS). Areas covered: In this review, we will focus on newly emerging DAC-high-yield process (HYP) therapy for MS. Based on published original articles and citable meeting abstracts, we will discuss its mode of action as well as data on efficacy and safety. Expert opinion: DAC has been observed to have multiple (biological) effects, which may contribute to beneficial effects in immune-related disease and particularly in relapsing-remitting MS. The positive results in the clinical studies represent achievement of an important milestone in the development of DAC-HYP as a potential new treatment option for MS patients. The benefit/risk ratios of this new biological agent in MS therapy are still being evaluated. Soon, DAC-HYP might qualify as MS therapy. A safety monitoring program is recommended in the clinical practice.
Via Krishan Maggon
Gastroenterology & Endoscopy News Study Hints Gut Microbiome Plays A Role in Multiple Sclerosis Gastroenterology & Endoscopy News The study included untreated MS patients (n=19) as well as those treated with interferon β-1a (n=21), interferon β-1b...
Via Krishan Maggon
Abstract Multiple sclerosis (MS) is a typical CD4 T cell-mediated autoimmune disease of the central nervous system (CNS) that leads to inflammation, demyelination, axonal damage, glial scarring and a broad range of neurological deficits. While disease-modifying drugs with a good safety profile and moderate efficacy have been available for 20 years now, a growing number of substances with superior therapeutic efficacy have recently been introduced or are in late stage clinical testing. Daclizumab, a humanized neutralizing monoclonal antibody against the α-chain of the Interleukin-2 receptor (IL-2Rα, CD25), which had originally been developed and approved to prevent rejection after allograft renal transplantation, belongs to the latter group. Clinical efficacy and safety of daclizumab in MS has so far been tested in several smaller phase II trials and recently two large phase II trials (combined 912 patients), and has shown efficacy regarding reduction of clinical disease activity as well as CNS inflammation. A phase III clinical trial is ongoing till March 2014 (DECIDE study, comparison with interferon (IFN) β-1a in RRMS). Furthermore, the existing safety data from clinical experience in kidney transplantation and in MS appears favorable. Apart from the promising clinical data mechanistic studies along the trials have provided interesting novel insights not only about the mechanisms of daclizumab treatment, but in general about the biology of IL-2 and IL-2 receptor interactions in the human immune system. Besides blockade of recently activated CD25+T cells daclizumab appears to act through additional mechanisms including the expansion of immune regulatory CD56bright natural killer (NK) cells, the blockade of cross-presentation of IL-2 by dendritic cells (DC) to T cells, and the reduction of lymphoid tissue inducer cells.
Via Krishan Maggon
Abstract Multiple sclerosis (MS) often results in chronic inflammatory and autoimmune disorders, and recent developments in understanding the disease pathogenesis has lead to newer therapeutic options for the treatment of the disease. The development of small molecule drugs with improved efficacy, better tolerability, and oral administration has received a new impetus with the discovery of newer classes of drugs. In this review, we have summarized the hitherto known synthetic strategies of fingolimod, laquinimod, cladribine, and teriflunomide reported in the literature which are the key small molecules and the first oral drug candidates for MS in various stages of clinical development or have been launched in the market.
Via Krishan Maggon
ABSTRACT Objective: This study evaluated the efficacy and safety of ATL1102, an antisense oligonucleotide that selectively targets the RNA for human CD49d, the α subunit of very late antigen 4, in patients with relapsing-remitting multiple sclerosis (RRMS). Methods: In a multicenter, double-blind, placebo-controlled randomized phase II trial, 77 patients with RRMS were treated with 200 mg of ATL1102 subcutaneously injected 3 times in the first week and twice weekly for 7 weeks or placebo and monitored for a further 8 weeks. MRI scans were taken at baseline and weeks 4, 8, 12, and 16. The primary endpoint was the cumulative number of new active lesions (either new gadolinium-enhancing T1 lesions or nonenhancing new or enlarging T2 lesions) at weeks 4, 8, and 12. Results: A total of 72 patients completed the study and 74 intention-to-treat patients were assessed. ATL1102 significantly reduced the cumulative number of new active lesions by 54.4% compared to placebo (mean 3.0 [SD 6.12] vs 6.2 [9.89], p = 0.01). The cumulative number of new gadolinium-enhancing T1 lesions was reduced by 67.9% compared to placebo (p = 0.002). Treatment-emergent adverse events included mild to moderate injection site erythema and decrease in platelet counts that returned to within the normal range after dosing. Conclusions: In patients with RRMS, ATL1102 significantly reduced disease activity after 8 weeks of treatment and was generally well-tolerated. This trial provides evidence for the first time that antisense oligonucleotides may be used as a therapeutic approach in neuroimmunologic disorders. Classification: This study provides Class I evidence that for patients with RRMS, the antisense oligonucleotide ATL1102 reduces the number of new active head MRI lesions.
Via Krishan Maggon
ABSTRACT Objective: To evaluate the safety, tolerability, and pharmacokinetics (PK) of BIIB033 (anti-LINGO-1 monoclonal antibody) in healthy volunteers and participants with multiple sclerosis (MS). Methods: In 2 separate randomized, placebo-controlled studies, single ascending doses (SAD; 0.1–100 mg/kg) of BIIB033 or placebo were administered via IV infusion or subcutaneous injection to 72 healthy volunteers, and multiple ascending doses (MAD; 0.3–100 mg/kg; 2 doses separated by 14 days) of BIIB033 or placebo were administered via IV infusion to 47 participants with relapsing-remitting or secondary progressive MS. Safety assessments included adverse event (AE) monitoring, neurologic examinations, conventional and nonconventional MRI, EEG, optical coherence tomography, retinal examinations, and evoked potentials. Serum and CSF PK as well as the immunogenicity of BIIB033 were also evaluated. Results: All 72 healthy volunteers and 47 participants with MS were included in the safety analyses. BIIB033 infusions were well tolerated. The frequency of AEs was similar between BIIB033 and placebo. There were no serious AEs or deaths. No clinically significant changes in any of the safety measures were observed. BIIB033 PK was similar between healthy volunteers and participants with MS. Doses of ≥10 mg/kg resulted in BIIB033 concentrations similar to or higher than the concentration associated with 90% of the maximum remyelination effect in rat remyelination studies. The incidence of anti-drug antibody production was low. Conclusions: The emerging safety, tolerability, and PK of BIIB033 support advancing BIIB033 into phase II clinical development as a potential treatment for CNS demyelination disorders. Classification of evidence: This study provides Class I evidence that BIIB033 is well tolerated and safe (serious adverse event rate 0%, 95% confidence interval 0–7.6%).
Via Krishan Maggon
Alemtuzumab for the treatment of relapsing-remitting multiple sclerosis: a review of its clinic pharmacology, ... http://t.co/GmAivJZDRp Multiple sclerosis (MS) is an inflammatory condition of the CNS presumably induced by an environmental trigger(s) in a genetically susceptible individual. Inflammation is prominent and most susceptible to intervention early in MS, so early treatment with disease-modifying therapies is recommended to reduce relapses and new MRI activity (both markers of inflammation) with the goal of delaying disability progression. Unfortunately, the response to the disease-modifying therapies is variable and often falls short of stopping observable disease activity, so the search for more effective agents continues. Alemtuzumab is a monoclonal antibody against CD52 that has exhibited significant efficacy throughout its clinical trial program in MS; uniquely, some of the studies have demonstrated a sustained reduction in disability in MS patients. Countering this impressive efficacy is an associated high risk of autoimmune events (especially thyroid) and concerns for infection or malignancy given prolonged immunosuppression after treatment with alemtuzumab.
Read More: http://informahealthcare.com/doi/abs/10.1586/1744666X.2014.951332
Via Krishan Maggon
Biogen Idec’s PLEGRIDY™(Peginterferon Beta-1a) Approved in the US for the Treatment of Multiple Sclerosis Reduces Relapses, Disability Progression and Brain Lesions with a Favorable Safety Profile − − Only Pegylated Interferon in MS, Dosed Once Every Two Weeks – − Complements Biogen Idec’s Industry-Leading Portfolio of MS Products – CAMBRIDGE, Mass.Today Biogen Idec (NASDAQ: BIIB) announced that the U.S. Food and Drug Administration (FDA) has approved PLEGRIDYTM (peginterferon beta-1a), a new treatment for people with relapsing forms of multiple sclerosis (RMS). PLEGRIDY, the only pegylated beta interferon approved for use in RMS, is dosed once every two weeks and can be administered subcutaneously with the PLEGRIDY PEN, a new, ready-to-use autoinjector, or a prefilled syringe. The FDA approval of PLEGRIDY is based on results from one of the largest pivotal studies of beta interferon conducted, ADVANCE, which involved more than 1,500 MS patients. ADVANCE was a two-year, Phase 3, placebo-controlled (in year one) study that evaluated the efficacy and safety of PLEGRIDY administered subcutaneously. The analysis for all primary and secondary efficacy endpoints occurred at the end of year one. After the first year, patients on placebo received PLEGRIDY for the duration of the study. In the first year of the ADVANCE clinical trial, PLEGRIDY dosed once every two weeks significantly reduced annualized relapse rate (ARR) at one year by 36 percent compared to placebo (p=0.0007). PLEGRIDY reduced the risk of 12-week confirmed disability progression, as measured by the Expanded Disability Status Scale, by 38 percent (p=0.0383) compared to placebo. PLEGRIDY also significantly reduced the number of new gadolinium-enhancing [Gd+] lesions by 86 percent (p<0.0001) and reduced new or newly enlarging T2-hyperintense lesions by 67 percent (p<0.0001) compared to placebo. The most common adverse reactions were injection site reaction, flu-like illness, fever, headache, muscle pain, chills, injection site pain, weakness, injection site itching and joint pain. The ADVANCE two-year safety data were consistent with safety results observed in year one.
Via Krishan Maggon
|
Abstract Myelin-reactive T cells have been identified in patients with multiple sclerosis (MS) and healthy subjects with comparable frequencies, but the contribution of these autoreactive T cells to disease pathology remains unknown. A total of 13,324 T cell libraries generated from blood of 23 patients and 22 healthy controls were interrogated for reactivity to myelin antigens. Libraries derived from CCR6+ myelin-reactive T cells from patients with MS exhibited significantly enhanced production of interferon-γ (IFN-γ), interleukin-17 (IL-17), and granulocyte-macrophage colony-stimulating factor (GM-CSF) compared to healthy controls. Single-cell clones isolated by major histocompatibility complex/peptide tetramers from CCR6+ T cell libraries also secreted more proinflammatory cytokines, whereas clones isolated from controls secreted more IL-10. The transcriptomes of myelin-specific CCR6+ T cells from patients with MS were distinct from those derived from healthy controls and, notably, were enriched in T helper cell 17 (TH17)–induced experimental autoimmune encephalitis gene signatures, and gene signatures derived from TH17 cells isolated other human autoimmune diseases. These data, although not causal, imply that functional differences between antigen-specific T cells from MS and healthy controls are fundamental to disease development and support the notion that IL-10 production from myelin-reactive T cells may act to limit disease progression or even pathogenesis.
Via Krishan Maggon
Abstract Multiple sclerosis (MS) is characterized by autoimmune inflammation affecting the central nervous system and subsequent neurodegeneration. Historically, damage was thought to be mediated exclusively by auto-antigen-activated pro-inflammatory T cells. However, more recently, we are gaining increasing knowledge on the pathogenic role played in MS by B cells, dendritic cells and monocytes. IFN-β therapy was one the first approved therapy for MS for its ability to reduce relapse rate and MRI lesion activity and to significantly decrease risk of disability progression. IFN-β-mediated mechanisms of action, even if not completely understood, mainly rely on its multifaceted pleiotropic effects resulting in sustained anti-inflammatory properties directed toward almost every immune cell type. Here, we will discuss in detail literature data characterizing the pathogenic activity of the different immune cell subsets involved in MS pathogenesis and how IFN-β therapy regulates their function by modulating bystander responses. We believe that the effectiveness of this drug in MS treatment, even if in use for a long time, can unveil new insights on this disease and still teach a lesson to researchers in the MS field.
Via Krishan Maggon
B cells are attracting increasing attention in the pathogenesis of multiple sclerosis (MS). B cell-targeted therapies with monoclonal antibodies or plasmapheresis have been shown to be successful in a subset of patients.
Via Krishan Maggon
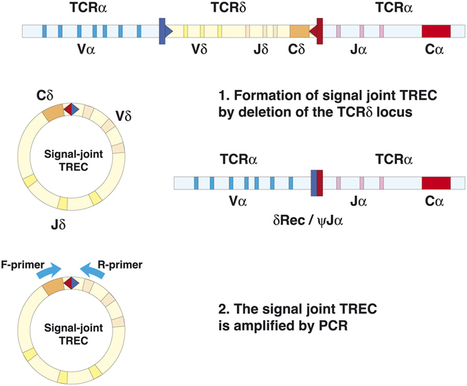
Abstract Background: Fingolimod inhibits lymphocyte egress from lymphoid tissues, thus altering the composition of the peripheral lymphocyte pool of multiple sclerosis patients. Objective: The objective of this paper is to evaluate whether fingolimod determines a decrease of newly produced T- and B-lymphocytes in the blood and a reduction in the T-cell receptor repertoire diversity that may affect immune surveillance. Methods: Blood samples were obtained from multiple sclerosis patients before fingolimod therapy initiation and then after six and 12 months. Newly produced T and B lymphocytes were measured by quantifying T-cell receptor excision circles and K-deleting recombination excision circles by real-time PCR, while recent thymic emigrants, naive CD8+ lymphocytes, immature and naive B cells were determined by immune phenotyping. T-cell receptor repertoire was analyzed by complementarity determining region 3 spectratyping. Results: Newly produced T and B lymphocytes were significantly reduced in peripheral blood of fingolimod-treated patients. The decrease was particularly evident in the T-cell compartment. T-cell repertoire restrictions, already present before therapy, significantly increased after 12 months of treatment. Conclusions: These results do not have direct clinical implications but they may be useful for further understanding the mode of action of this immunotherapy for multiple sclerosis patients.
Via Krishan Maggon
Highlights • Ruth Arnon and Michael Sela profoundly influenced the development of a model system to test new therapies in multiple sclerosis. • By measuring clinical, pathologic, and immunologic outcomes, the biological implications of new drugs could be elucidated. • The pioneering research on Copaxone using the EAE model, paved the way for the discovery of other therapeutics in MS.
Via Krishan Maggon
RT @MSDForum: Daclizumab did well in phase 3 trials for #MS. Here's the story behind the story http://t.co/PFzsfHqKs1 an analysis of samples from blood and cerebrospinal fluid suggests that daclizumab nudges the abnormal numbers of innate and adaptive immune cells in RRMS back to more normal physiological levels found in people without MS (Lin et al., 2014). the therapeutic efficacy of daclizumab paralleled the expansion of a key target cell population, called CD56bright natural killer cells (CD56bright NK). That raised hopes for a biomarker that could measure who was responding and predict who would do best.
Via Krishan Maggon
Background and purpose Susceptibility to multiple sclerosis (MS) is determined by environmental and genetic factors, but the cause remains unknown. Changes to the proteome prior to first symptom onset may reflect the underlying pathophysiology of the disease. Methods This preliminary study utilized pre-symptomatic and post-symptomatic serum from a sample of 100 incident population-based US military veterans with MS along with 100 matched healthy controls. All samples were obtained from the Department of Defense Serum Repository. Multidimensional protein identification technology tandem mass spectrometry analysis was performed on tryptic peptides of lectin-captured glycosylated serum proteins following albumin/immunoglobulin G depletion. Identified proteins were analyzed with the Ingenuity Pathway Analysis program. Results The mean intervals between first symptom onset and the collection of pre-symptomatic and post-symptomatic sera were −6.0 and +1.1 years, respectively. Pre-symptomatic proteins from the MS group were differentially regulated compared with both control groups indicating that proteomic changes are detected prior to symptom onset. Pathway analysis showed that proteins involved in the complement and coagulation pathways and lipid transport are significantly altered in the serum of subjects with MS compared with healthy donors. Conclusions Compared with healthy controls, differential proteomic changes were noted in the serum of patients with MS that preceded the onset of symptomatic disease. Further work is in progress to confirm or refute these findings.
Via Krishan Maggon
|


 Your new post is loading...
Your new post is loading...