Alemtuzumab for the treatment of relapsing-remitting multiple sclerosis: a review of its clinic pharmacology, ... http://t.co/GmAivJZDRp
Multiple sclerosis (MS) is an inflammatory condition of the CNS presumably induced by an environmental trigger(s) in a genetically susceptible individual. Inflammation is prominent and most susceptible to intervention early in MS, so early treatment with disease-modifying therapies is recommended to reduce relapses and new MRI activity (both markers of inflammation) with the goal of delaying disability progression. Unfortunately, the response to the disease-modifying therapies is variable and often falls short of stopping observable disease activity, so the search for more effective agents continues. Alemtuzumab is a monoclonal antibody against CD52 that has exhibited significant efficacy throughout its clinical trial program in MS; uniquely, some of the studies have demonstrated a sustained reduction in disability in MS patients. Countering this impressive efficacy is an associated high risk of autoimmune events (especially thyroid) and concerns for infection or malignancy given prolonged immunosuppression after treatment with alemtuzumab.
Read More: http://informahealthcare.com/doi/abs/10.1586/1744666X.2014.951332
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...



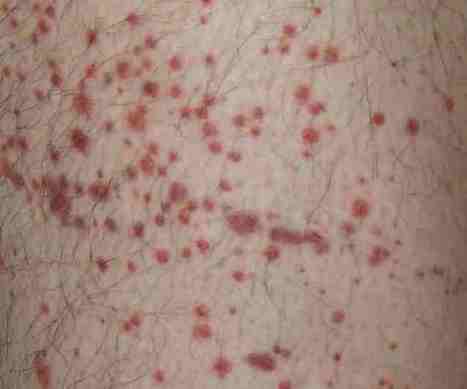






Posted online on August 22, 2014. (doi:10.1586/1744666X.2014.951332)David E Jones and Myla D Goldman *Department of Neurology, James Q. Miller MS Clinic, University of Virginia Health System, 500 Ray C. Hunt Drive, Charlottesville, VA 22908, USA*Author for correspondence: mdg3n@virginia.eduRead More: http://informahealthcare.com/doi/abs/10.1586/1744666X.2014.951332