Your new post is loading...
 Your new post is loading...

|
Scooped by
HAS-veille
|
Background
Despite childhood vaccine mandates imposed in 2018 in France, parental vaccine hesitancy (VH) remains frequent. Interventions in Quebec, Canada, applying motivational interviewing (MI) techniques have successfully reduced parents’ VH for childhood immunisations.
Aim
To determine whether MI intervention for mothers in maternity wards in the days after birth in France could significantly reduce VH, increase intentions to vaccinate (VI) their child at 2 months and reduce VH social inequalities.
Methods
We conducted a parallel-arm multicentre randomised controlled trial from November 2021 to April 2022 to compare impacts of MI performed by MI-trained midwives (intervention) vs a vaccination leaflet (control). We included 733 mothers from two maternity hospitals in south-eastern France, randomly assigned either arm. The validated Parents Attitudes about Childhood Vaccines questionnaire was used before and after MI or leaflet to assess mothers’ VH (13 items, 0–100 score) and VI (1 item, 1–10 score). Difference-in-difference (D-I-D) models were used to estimate net impact of MI vs leaflet for the entire sample and stratified by VH and education level.
Results
Motivational interview intervention reduced mothers' VH score by 33% (p < 0.0001) and increased VI by 8% (p < 0.0001); the effect was largest for the highest initial VH levels. D-I-D analyses estimated net VH decrease at 5.8/100 points (p = 0.007) and net VI increase at 0.6/10 points (p = 0.005). Net VH decrease was highest for high initial VH levels and low education levels.
Conclusions
Our results show positive effects of MI intervention, and means of its implementation should be investigated in France.

|
Scooped by
HAS-veille
|
mRNA vaccines have emerged as highly effective strategies in the prophylaxis and treatment of diseases, thanks largely although not totally to their extraordinary performance in recent years against the worldwide plague COVID-19. The huge superiority of mRNA vaccines regarding their efficacy, safety, and large-scale manufacture encourages pharmaceutical industries and biotechnology companies to expand their application to a diverse array of diseases, despite the nonnegligible problems in design, fabrication, and mode of administration. This review delves into the technical underpinnings of mRNA vaccines, covering mRNA design, synthesis, delivery, and adjuvant technologies. Moreover, this review presents a systematic retrospective analysis in a logical and well-organized manner, shedding light on representative mRNA vaccines employed in various diseases. The scope extends across infectious diseases, cancers, immunological diseases, tissue damages, and rare diseases, showcasing the versatility and potential of mRNA vaccines in diverse therapeutic areas. Furthermore, this review engages in a prospective discussion regarding the current challenge and potential direction for the advancement and utilization of mRNA vaccines. Overall, this comprehensive review serves as a valuable resource for researchers, clinicians, and industry professionals, providing a comprehensive understanding of the technical aspects, historical context, and future prospects of mRNA vaccines in the fight against various diseases.

|
Scooped by
HAS-veille
|
COVID-19 vaccines with alternative strain compositions are needed to provide broad protection against newly emergent SARS-CoV-2 variants of concern. T…

|
Scooped by
HAS-veille
|
AbstractBackgrounds. Knowing the duration of effectiveness of COVID-19 booster doses is essential to provide decision makers with scientific arguments about the

|
Scooped by
HAS-veille
|
This cohort study compares the reactogenicity and immunogenicity of COVID-19 and influenza vaccinations administered together with those of COVID-19 vaccination alone.

|
Scooped by
HAS-veille
|
The objective of this study was to estimate and compare the cost-effectiveness of switching from a bivalent to a nonavalent human papillomavirus (HPV) vaccination program in Norway, incorporatin

|
Scooped by
HAS-veille
|
This report describes ACIP recommendations for the use of pneumococcal vaccine in U.S. adults.

|
Scooped by
HAS-veille
|
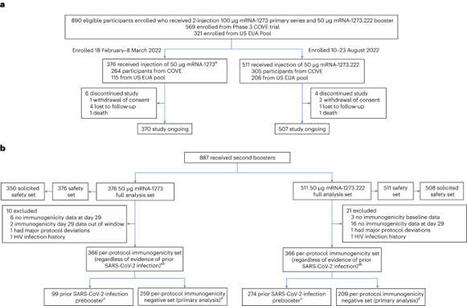
This ongoing, open-label, phase 2/3 trial compared the safety and immunogenicity of the Omicron BA.4/BA.5-containing bivalent mRNA-1273.222 vaccine with the ancestral Wuhan-Hu-1 mRNA-1273 as booster doses. Two groups of adults who previously received mRNA-1273 as primary vaccination series and booster doses were enrolled in a sequential, nonrandomized manner and received single-second boosters of mRNA-1273 (n = 376) or bivalent mRNA-1273.222 (n = 511). Primary objectives were safety and the noninferiority or superiority of neutralizing antibody (nAb) responses against Omicron BA.4/BA.5 and ancestral SARS-CoV-2 with the D614G mutation (ancestral SARS-CoV-2 (D614G)), 28 days post boost. Superiority and noninferiority were based on prespecified success criteria (lower bounds of 95% CI > 1 and < 0.677, respectively) of the mRNA-1273.222:mRNA-1273 geometric mean ratios. Bivalent Omicron BA.4/BA.5-containing mRNA-1273.222 elicited superior nAb responses against BA.4/BA.5 versus mRNA-1273 and noninferior responses against ancestral SARS-CoV-2 (D614G) at day 29 post boost in participants without detectable prior SARS-CoV-2 infection. Day 29 seroresponses against Omicron BA.4/BA.5 were higher for mRNA-1273.222 than for mRNA-1273 and similar against ancestral SARS-CoV-2 (D614G), both meeting noninferiority criterion. The safety profile of mRNA-1273.222 was similar to that previously reported for mRNA-1273 with no new safety concerns identified. Continued monitoring of neutralization and real-world vaccine effectiveness are needed as additional divergent-virus variants emerge. ClinicalTrials.gov registration: NCT04927065. An Omicron BA.4/BA.5 mRNA booster vaccine elicits high neutralizing responses to the BA.4/BA.5 variant and to ancestral SARS-CoV-2, supporting tailoring booster vaccines to the predominant Omicron variant.

|
Scooped by
HAS-veille
|
In tuberculosis (TB) vaccine development, multiple factors hinder the design and interpretation of the clinical trials used to estimate vaccine efficacy. The complex transmission chain of TB includes multiple routes to disease, making it hard to link the vaccine efficacy observed in a trial to specific protective mechanisms. Here, we present a Bayesian framework to evaluate the compatibility of different vaccine descriptions with clinical trial outcomes, unlocking impact forecasting from vaccines whose specific mechanisms of action are unknown. Applying our method to the analysis of the M72/AS01E vaccine trial -conducted on IGRA+ individuals- as a case study, we found that most plausible models for this vaccine needed to include protection against, at least, two over the three possible routes to active TB classically considered in the literature: namely, primary TB, latent TB reactivation and TB upon re-infection. Gathering new data regarding the impact of TB vaccines in various epidemiological settings would be instrumental to improve our model estimates of the underlying mechanisms. The complex transmission chain of tuberculosis (TB) forces mathematical modelers to make mechanistic assumptions when modelling vaccine effects. Here, authors posit a Bayesian formalism that unlocks mechanism-agnostic impact forecasts for TB vaccines.

|
Scooped by
HAS-veille
|

|
Scooped by
HAS-veille
|
The COVID-19 pandemic has challenged traditional vaccine guidance infrastructure and frameworks, and added urgency and complexity to the operation of …

|
Scooped by
HAS-veille
|
Background Influenza infection is a highly preventable transmissible viral disease associated with mild upper respiratory symptoms and more severe conditions such as lethal pneumonia. Studies have shown that a broader spectrum influenza vaccine could reduce influenza’s burden of disease in low- and middle-income countries. A considerable number of systematic reviews reported that quadrivalent influenza vaccines are considered more effective compared to trivalent vaccines, hence, there is a need for an overview in order to synthesize the current evidence pertaining to the comparison between quadrivalent and trivalent inactivated influenza vaccines. Objective: The aim was to summarize the evidence from systematic reviews that investigated the immunogenicity and safety of the Influenza’s inactivated quadrivalent vaccine (QIV) compared to the trivalent vaccine (TIV), in the general population. Methods We searched articles up to December 2022 at: Web of Science, EMBASE, MEDLINE, Cochrane Library, and SCOPUS. The search strategy was conducted following the PICO model. We included systematic reviews comparing the primary outcomes of immunogenicity (seroprotection rate and seroconversion rate) and adverse events using risk ratios. The AMSTAR 2 and ROBIS were used for quality assessments, and GRADE was used for evidence certainty assessments. Findings We included five systematic reviews, totalling 47,740 participants. The Quadrivalent Inactivated Influenza Vaccine (QIV) exhibited enhanced immunogenicity in the context of B-lineage mismatch when compared to the Trivalent Inactivated Influenza Vaccine (TIV). While the safety profile of QIV was found to be comparable to that of TIV, the QIV showed a higher incidence of solicited local pain among children and adolescents, as well as an increased frequency of local adverse events within the adult population. Conclusion Our findings suggest that the QIV provides a superior immunogenicity response compared to the TIV in all age groups evaluated, especially when a lineage mismatch occurred. The safety of QIV was considered similar to the TIV, with no serious or systemic solicited or unsolicited adverse events; tough pain at the injection site was greater for QIV. We recommend caution owing to the high risk of bias in the selection process and no protocol registration.

|
Scooped by
HAS-veille
|
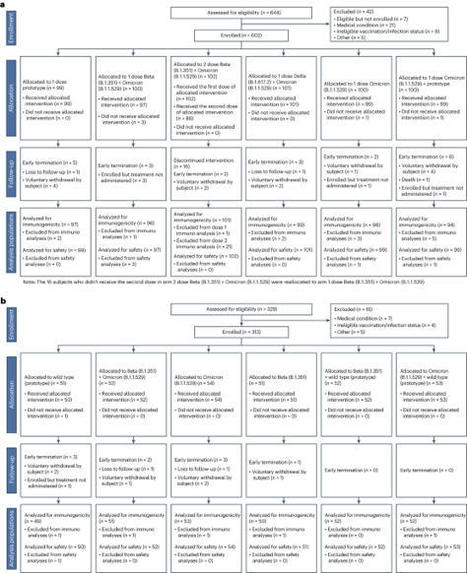
Vaccine protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection wanes over time, requiring updated boosters. In a phase 2, open-label, randomized clinical trial with sequentially enrolled stages at 22 US sites, we assessed safety and immunogenicity of a second boost with monovalent or bivalent variant vaccines from mRNA and protein-based platforms targeting wild-type, Beta, Delta and Omicron BA.1 spike antigens. The primary outcome was pseudovirus neutralization titers at 50% inhibitory dilution (ID50 titers) with 95% confidence intervals against different SARS-CoV-2 strains. The secondary outcome assessed safety by solicited local and systemic adverse events (AEs), unsolicited AEs, serious AEs and AEs of special interest. Boosting with prototype/wild-type vaccines produced numerically lower ID50 titers than any variant-containing vaccine against all variants. Conversely, boosting with a variant vaccine excluding prototype was not associated with decreased neutralization against D614G. Omicron BA.1 or Beta monovalent vaccines were nearly equivalent to Omicron BA.1 + prototype or Beta + prototype bivalent vaccines for neutralization of Beta, Omicron BA.1 and Omicron BA.4/5, although they were lower for contemporaneous Omicron subvariants. Safety was similar across arms and stages and comparable to previous reports. Our study shows that updated vaccines targeting Beta or Omicron BA.1 provide broadly crossprotective neutralizing antibody responses against diverse SARS-CoV-2 variants without sacrificing immunity to the ancestral strain. ClinicalTrials.gov registration: NCT05289037 . Analysis of antibody responses to COVID-19 vaccines encoding variant-specific spike, with or without ancestral spike, suggests no loss of neutralization of the ancestral virus with variant-only vaccines, which may simplify future vaccine updates.
|

|
Scooped by
HAS-veille
|
Vaccine-preventable diseases continue to contribute to increased morbidity and mortality, even in high-income countries where vaccines are easily accessible. A reason for this is suboptimal uptak

|
Scooped by
HAS-veille
|
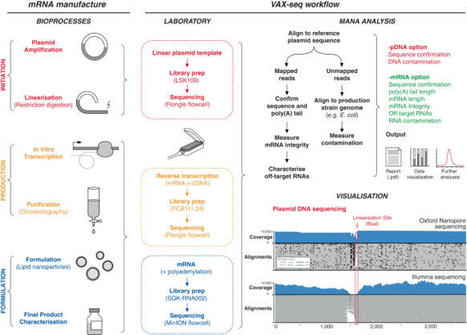
The success of mRNA vaccines has been realised, in part, by advances in manufacturing that enabled billions of doses to be produced at sufficient quality and safety. However, mRNA vaccines must be rigorously analysed to measure their integrity and detect contaminants that reduce their effectiveness and induce side-effects. Currently, mRNA vaccines and therapies are analysed using a range of time-consuming and costly methods. Here we describe a streamlined method to analyse mRNA vaccines and therapies using long-read nanopore sequencing. Compared to other industry-standard techniques, VAX-seq can comprehensively measure key mRNA vaccine quality attributes, including sequence, length, integrity, and purity. We also show how direct RNA sequencing can analyse mRNA chemistry, including the detection of nucleoside modifications. To support this approach, we provide supporting software to automatically report on mRNA and plasmid template quality and integrity. Given these advantages, we anticipate that RNA sequencing methods, such as VAX-seq, will become central to the development and manufacture of mRNA drugs. mRNA vaccines must be rigorously analysed to measure their integrity and detect contaminants, which can be time-consuming and costly. Here, authors describe a method to analyse mRNA vaccine quality using long-read sequencing and a custom bioinformatic pipeline.

|
Scooped by
HAS-veille
|

|
Scooped by
HAS-veille
|
Facts, data, and analytics about biomedical matters.

|
Scooped by
HAS-veille
|

|
Scooped by
HAS-veille
|
This study describes the evolution of vaccination acceptability and associated determinants in the French general population between 2000 and 2021, an…

|
Scooped by
HAS-veille
|
Couverture vaccinale contre la grippe des femmes enceintes, propositions de vaccination et étude des déterminants, France métropolitaine, 2019-2021

|
Scooped by
HAS-veille
|
: Human behavior and more specifically behavioral insight-based approaches to vaccine uptake have often been overlooked. While there have been a few n…

|
Scooped by
HAS-veille
|
A vaccine that prevents cytomegalovirus (CMV) infection in women could reduce the incidence of congenital CMV infection, a major cause of neurodevelop…

|
Scooped by
HAS-veille
|
Countries routinely offering acellular pertussis vaccine, where long-term protection is not sustained, have the challenge of selecting an optimal sche…

|
Scooped by
HAS-veille
|
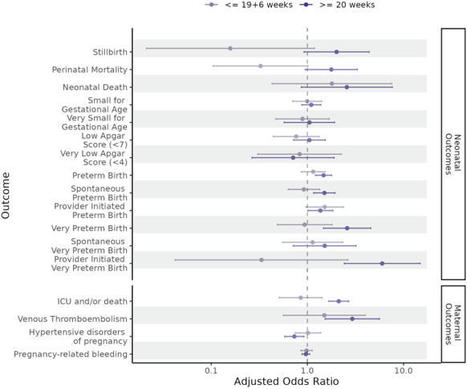
Understanding the impact of SARS-CoV-2 infection and COVID-19 vaccination in pregnancy on neonatal and maternal outcomes informs clinical decision-making. Here we report a national, population-based, matched cohort study to investigate associations between SARS-CoV-2 infection and, separately, COVID-19 vaccination just before or during pregnancy and the risk of adverse neonatal and maternal outcomes among women in Scotland with a singleton pregnancy ending at ≥20 weeks gestation. Neonatal outcomes are stillbirth, neonatal death, extended perinatal mortality, preterm birth (overall, spontaneous, and provider-initiated), small-for-gestational age, and low Apgar score. Maternal outcomes are admission to critical care or death, venous thromboembolism, hypertensive disorders of pregnancy, and pregnancy-related bleeding. We use conditional logistic regression to derive odds ratios adjusted for socio-demographic and clinical characteristics (aORs). We find that infection is associated with an increased risk of preterm (aOR=1.36, 95% Confidence Interval [CI] = 1.16–1.59) and very preterm birth (aOR = 1.90, 95% CI 1.20–3.02), maternal admission to critical care or death (aOR=1.72, 95% CI = 1.39–2.12), and venous thromboembolism (aOR = 2.53, 95% CI = 1.47–4.35). We find no evidence of increased risk for any of our outcomes following vaccination. These data suggest SARS-CoV-2 infection during pregnancy is associated with adverse neonatal and maternal outcomes, and COVID-19 vaccination remains a safe way for pregnant women to protect themselves and their babies against infection. The impacts of SARS-CoV-2 infection and COVID-19 vaccination in pregnancy are not fully understood. Here, the authors perform a cohort study using data from Scotland and find that infection was associated with increased risk of preterm birth and some adverse maternal outcomes, but there was no evidence of adverse outcomes associated with vaccination.

|
Scooped by
HAS-veille
|
Background To eliminate cervical cancer as a public health problem, the World Health Organization had recommended routine vaccination of adolescent girls with two doses of the human papillomavirus (HPV) vaccine before sexual initiation. However, many countries have yet to implement HPV vaccination because of financial or logistical barriers to delivering two doses outside the infant immunisation programme. Methods Using three independent HPV transmission models, we estimated the long-term health benefits and cost-effectiveness of one-dose versus two-dose HPV vaccination, in 188 countries, under scenarios in which one dose of the vaccine gives either a shorter duration of full protection (20 or 30 years) or lifelong protection but lower vaccine efficacy (e.g. 80%) compared to two doses. We simulated routine vaccination with the 9-valent HPV vaccine in 10-year-old girls at 80% coverage for the years 2021–2120, with a 1-year catch-up campaign up to age 14 at 80% coverage in the first year of the programme. Results Over the years 2021–2120, one-dose vaccination at 80% coverage was projected to avert 115.2 million (range of medians: 85.1–130.4) and 146.8 million (114.1–161.6) cervical cancers assuming one dose of the vaccine confers 20 and 30 years of protection, respectively. Should one dose of the vaccine provide lifelong protection at 80% vaccine efficacy, 147.8 million (140.6–169.7) cervical cancer cases could be prevented. If protection wanes after 20 years, 65 to 889 additional girls would need to be vaccinated with the second dose to prevent one cervical cancer, depending on the epidemiological profiles of the country. Across all income groups, the threshold cost for the second dose was low: from 1.59 (0.14–3.82) USD in low-income countries to 44.83 (3.75–85.64) USD in high-income countries, assuming one dose confers 30-year protection. Conclusions Results were consistent across the three independent models and suggest that one-dose vaccination has similar health benefits to a two-dose programme while simplifying vaccine delivery, reducing costs, and alleviating vaccine supply constraints. The second dose may become cost-effective if there is a shorter duration of protection from one dose, cheaper vaccine and vaccination delivery strategies, and high burden of cervical cancer.
|



 Your new post is loading...
Your new post is loading...