 Your new post is loading...

|
Scooped by
Juan Lama
|
Aging, or senescent cells, which stop dividing but don’t die, can accumulate in the body over the years and fuel chronic inflammation that contributes to conditions such as cancer and degenerative disorders. In mice, eliminating senescent cells from aging tissues can restore tissue balance and lead to an increased healthy lifespan. Now a team led by investigators at Massachusetts General Hospital (MGH), a founding member of Mass General Brigham (MGB), has found that the immune response to a virus that is ubiquitously present in human tissues can detect and eliminate senescent cells in the skin. For the study, which is published in Cell, the scientists analyzed young and old human skin samples to learn more about the clearance of senescent cells in human tissue. The researchers found more senescent cells in the old skin compared with young skin samples. However, in the samples from old individuals, the number of senescent cells did not increase as individuals got progressively older, suggesting that some type of mechanism kicks in to keep them in check. Experiments suggested that once a person becomes elderly, certain immune cells called killer CD4+ T cells are responsible for keeping senescent cells from increasing. Indeed, higher numbers of killer CD4+ T cells in tissue samples were associated with reduced numbers of senescent cells in old skin. When they assessed how killer CD4+ T cells keep senescent cells in check, the researchers found that aging skin cells express a protein, or antigen, produced by human cytomegalovirus, a pervasive herpesvirus that establishes lifelong latent infection in most humans without any symptoms. By expressing this protein, senescent cells become targets for attack by killer CD4+ T cells. “Our study has revealed that immune responses to human cytomegalovirus contribute to maintaining the balance of aging organs,” says senior author Shawn Demehri, MD, PhD, director of the High Risk Skin Cancer Clinic at MGH and an associate professor of Dermatology at Harvard Medical School. “Most of us are infected with human cytomegalovirus, and our immune system has evolved to eliminate cells, including senescent cells, that upregulate the expression of cytomegalovirus antigens.” These findings, which highlight a beneficial function of viruses living in our body, could have a variety of clinical applications. “Our research enables a new therapeutic approach to eliminate aging cells by boosting the anti-viral immune response,” says Demehri. “We are interested in utilizing the immune response to cytomegalovirus as a therapy to eliminate senescent cells in diseases like cancer, fibrosis and degenerative diseases.” Demehri notes that the work may also lead to advances in cosmetic dermatology, for example in the development of new treatments to make skin look younger. Co-authors include Tatsuya Hasegawa, Tomonori Oka, Heehwa G. Son, Valeria S. Oliver-García, Marjan Azin, Thomas M. Eisenhaure, David J. Lieb, and Nir Hacohen. This study was supported by the Burroughs Wellcome Fund and Shiseido Co. Ltd. Study Published in Cell (March 30, 2023): https://doi.org/10.1016/j.cell.2023.02.033

|
Scooped by
Juan Lama
|
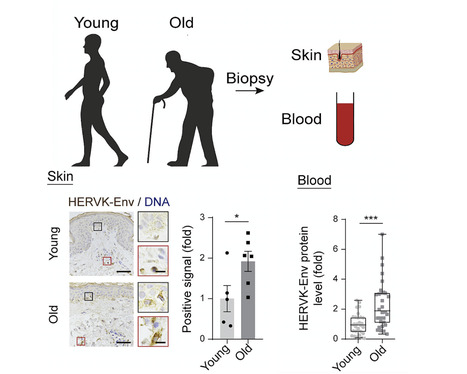
Liu and colleagues uncover the ways in which derepression of human endogenous retrovirus triggers cellular senescence and tissue aging; the findings provide fresh insights into therapeutic strategies for alleviating aging. Summary Whether and how certain transposable elements with viral origins, such as endogenous retroviruses (ERVs) dormant in our genomes, can become awakened and contribute to the aging process is largely unknown. In human senescent cells, we found that HERVK (HML-2), the most recently integrated human ERVs, are unlocked to transcribe viral genes and produce retrovirus-like particles (RVLPs). These HERVK RVLPs constitute a transmissible message to elicit senescence phenotypes in young cells, which can be blocked by neutralizing antibodies. The activation of ERVs was also observed in organs of aged primates and mice as well as in human tissues and serum from the elderly. Their repression alleviates cellular senescence and tissue degeneration and, to some extent, organismal aging. These findings indicate that the resurrection of ERVs is a hallmark and driving force of cellular senescence and tissue aging. Highlights - Derepression of the endogenous retrovirus contributes to programmed aging
- Upregulation of HERVK triggers the innate immune response and cellular senescence
- Extracellular HERVK retrovirus-like particles induce senescence in young cells
- Endogenous retrovirus serves as a potential target to alleviate aging
Published in Cell (Jan. 6, 2023): https://doi.org/10.1016/j.cell.2022.12.017

|
Scooped by
Juan Lama
|
First-in-human study aims to deliver viral vectors with hTERT gene in five individuals aged 45+. Clinical trial (NCT04133649) just began recruitment stage. The procedure will consist of a single intravenous injection, followed by six safety and efficacy evaluations. Its author, Libella Gene Therapeutics, declares: The goal is to extend the telomeres to prevent, delay, or even reverse Aging. Participants will receive adeno-associated virus (AAV) containing gene expressing telomerase reverse transcriptase enzyme. AAV is expected to move from the circulatory system to tissues, invade cells, and establish telomerase expression inside cells. Viruses will not modify the genome – AAV’s genetic material normally exists separately in the cell cytoplasm (as an episome). There are two key aspects to consider the potential of this approach: - How efficient AAV will be in reaching organs crucial for aging and establishing telomerase expression inside cells? AAV reaches less than 0.2% muscle cells, including heart cells. Not all AAV serotypes cross blood-brain barrier and typically they required special optimization to spread through brain. For high efficiency we need high doses, but their upper range is limited by immunological reactions.
- How efficient is telomerase expression in anti-aging approach? Experiments on mice show hat telomerase can extend their median survival by 20%. However, those results are not consistent between species and therefore may not be extendable to humans. Recent through review of animal studies in longevity gave relatively little attention to the shortening of telomeres, arguing that multiple other mechanisms are more crucial in aging.
Formally, the study is phase I trial, which limits the main goal – whether it is successful or failed attempt – to safety only. In this case, the primary goal was declared as the incidence of adverse effects. Determination of dosing and tolerability is an important first step in all gene therapies. High doses of viral particles result in significant immunological reaction. Moreover, liver damage is a common adverse effect in early gene therapies, because of liver’s participation in blood filtering. In addition, telomerase introduces additional risk on its own. In 85% cases of cancers, telomerase is found upregulated, which raises concerns about potential oncogenicity of AAVs with hTERT gene. Secondary goals include measurement of telomerase activity, telomere length, and general physical exams. The trial is accompanied by two similar phase I attempts (NCT04133454, NCT04110964), which target Alzheimer’s disease and critical limb ischemia. According to third-party information, procedures will be conducted in South America: Patients participating in the trial will be enrolled in their country of origin and will travel to Colombia. Patients will stay in Colombia for a few days while the treatment is administered and hospitalized for observation. Patients will then return to their country of origin and will be followed-up per the study protocol. Press Release published December 6, 2019 available here: https://www.prnewswire.com/news-releases/libella-gene-therapeutics-announces-experimental-research-project-in-hopes-of-reversing-alzheimers-300567791.html
|

|
Scooped by
Juan Lama
|
But doubts remain about whether cell reprogramming technique could one day help. A decade after Kyoto University biologist Shinya Yamanaka won a share of a Nobel Prize for discovering a cocktail of proteins that reprogram adult cells into versatile stem cells, two teams argue the proteins can turn back the clock for entire organisms—perhaps one day humans. One group at a biotech used gene therapy to deliver some of the so-called Yamanaka factors into old mice, and modestly extended their life span. And a separate team followed a similar strategy to reverse aging-like changes in genetically engineered mice. In both cases, the Yamanaka factors appear to have restored part of the animals’ epigenome, chemical modifications on DNA and proteins that help regulate gene activity, to a more youthful state. But scientists not involved in the work say suggestions of age reversal are premature. “These studies use reprogramming factors to reverse epigenetic changes that happen during aging,” says Matt Kaeberlein, a geroscientist at the University of Washington, Seattle, but that’s a far cry from making an old animal young again. Several groups had already found genetically engineered mice that begin expressing Yamanaka factors in adulthood show reversal of certain aging symptoms. To explore an approach that might lead to a more practical treatment for people, San Diego–based company Rejuvenate Bio injected elderly (124-week-old) mice with adeno-associated viruses (AAVs) carrying genes for three of the factors, collectively known as OSK. These animals lived another 18 weeks on average, compared with 9 weeks for a control group, the company reported in a preprint on bioRxiv this month. They also partially regained patterns of DNA methylation—a type of epigenetic mark—typical of younger animals. Although some studies have suggested Yamanaka factors can promote cancer, Noah Davidsohn, Rejuvenate’s chief scientific officer and co-founder, says the company has so far found no obvious negative effects in mice given the gene therapy. “I would say it is provocative—possibly a breakthrough,” says Steven Austad of the University of Alabama, Birmingham, who studies the biology of aging. “But it will need to be replicated and the mechanism explored before we can say for sure.” The second study, published yesterday in Cell, is from a team led by Harvard Medical School geneticist David Sinclair, who has backed several controversial “antiaging” interventions over the past 2 decades. (Rejuvenate’s approach grew from an earlier collaboration between Sinclair and Davidsohn, but Sinclair isn’t involved in the company’s research, Davidsohn says.) Sinclair’s team set out to test his “information theory of aging,” which posits that our bodies get old because of the cumulative loss of epigenetic marks. Cells’ DNA repair mechanisms, operating throughout a lifetime to fix DNA cuts and other damage, are what degrade these marks, he argues. To test the theory in mammals, the team genetically engineered a mouse strain that, when given a particular drug, makes an enzyme that cuts their DNA at 20 sites in the genome, which are then faithfully repaired. Widespread changes in cells’ DNA methylation patterns and gene expression followed, consistent with Sinclair’s theory. The mice ended up with an epigenetic signature more like that of older animals, and their health deteriorated. Within weeks, they lost hair and pigment; within months, they showed multiple signs of frailty and tissue aging. To see whether the epigenetic degradation was reversible, the researchers injected some of these elderly seeming mice with AAVs carrying OSK genes, which Sinclair’s group recently reported could reverse loss of vision in aging rodents. Analyses of the mice’s muscles, kidneys, and retinas suggest the cocktail reversed some of the epigenetic changes induced by the DNA breaks. The findings point to a way to drive an animal’s age “forwards and backwards at will,” Sinclair says, and support the idea of epigenome-targeting treatments for aging in humans. Molecular biologist Wolf Reik, director of the Altos Cambridge Institute of Science (opened last year by rejuvenation-focused company Altos Labs), praised the sophistication and thoroughness of the Harvard team’s study, but says the team’s indirect way of inducing epigenetic changes with dramatic DNA breaks that could have other effects makes it hard to prove those changes are what’s causing aging. It’s also unclear how well mice with induced DNA breaks mimic naturally aging animals, says Jan Vijg, a geneticist at the Albert Einstein College of Medicine. He and others stress that aging is a complex process with multiple contributing factors, and that in both papers, the effects of OSK treatment were moderate: a small extension of life span in one, and a partial reversal of artificially induced symptoms in the other. “The jump that now aging is a program” that can be wound backward isn’t justified by the research, Vijg says. Still, both groups want to move their work toward the clinic. Rejuvenate is examining the mechanisms underlying the treatment’s action and tweaking its delivery and composition, Davidsohn says. “OSK might not be the final set” of factors, he adds. Sinclair says his team is already testing AAV-delivered OSK in the eyes of monkeys. “If those studies in monkeys go well and everything looks safe enough for humans, the plan is to immediately apply to the FDA [Food and Drug Administration] to do a study in one or more [age-related] diseases of blindness.” Published in Science (Jan.13, 2023): https://doi.org/10.1126/science.adg6801 See also: https://doi.org/10.1101/2023.01.04.522507 https://doi.org/10.1016/j.cell.2022.12.027

|
Scooped by
Juan Lama
|
Aging is associated with increased morbidity arising from a range of tissue dysfunctions. A common denominator of age-associated frailty is increased baseline inflammation, called inflammaging, that is present in older individuals. Recent studies have shown that the presence of excessive inflammation can inhibit immunity in both animals and humans and that this can be prevented by blocking inflammatory processes. This finding has important implications for the immunity of older individuals who are infected with pathogens such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that induce overwhelming inflammation, which can be fatal, particularly in older people. Reducing inflammation may be a therapeutic strategy for enhancing immunity in older people. SARS-CoV-2 causes severe respiratory disease (coronavirus disease 2019, COVID-19) that mostly induces mild to moderate symptoms in younger individuals, but induces devastating morbidity and mortality in older individuals. A key hallmark of severe disease is exuberant inflammation in the respiratory tract of patients. Older healthy individuals (60 years and above) exhibit chronic low-grade sterile inflammation (not caused by a pathogen) characterized by high baseline serum concentrations of C reactive protein (CRP) and cytokines, including interleukin-6 (IL-6), and IL-8. This inflammaging predicts frailty and earlier mortality compared with individuals of the same age group who do not exhibit increased baseline systemic inflammation. Inflammaging may arise as a result of multiple mechanisms, including the accumulation of misfolded proteins, compromised gut barrier function, and obesity...... Original publication in Science (July 17, 2020): https://doi.org/10.1126/science.abb0762
|



 Your new post is loading...
Your new post is loading...










Buy Klonopin without prescription
buy liquid morphine online
Buy Morphine online uk
Buy Norco Online Without Prescription
Buy Oramorph without prescription
Buy oxycontin online uk
Buy Quaaludes Online
buy sildenafil 50 mg tablet
Buy yellow Xanax online
Dilaudid for pain
Order Acxion phentermine 30mg
Order codeine without prescription