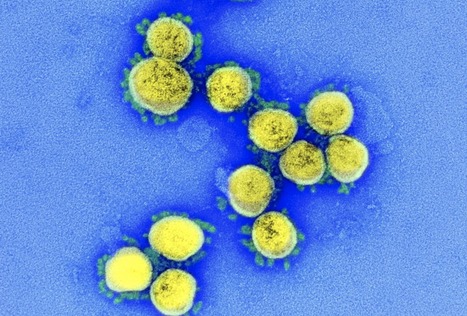
Researchers in Europe and the United States have demonstrated the potential of the influenza drug enisamium as a treatment for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19). Aartjan te Velthuis from the University of Cambridge in the UK and colleagues showed that enisamium prevented SARS-CoV-2 replication in human cell lines and stopped viral RNA synthesis in vitro. Furthermore, in a double-blind, randomized, placebo-controlled trial of adults hospitalized with COVID-19, enisamium significantly reduced recovery time among patients who required supplementary oxygen. The drug is already clinically approved for use against influenza in eleven different countries. Furthermore, enisamium does not require intravenous administration and could be used outside of the hospital setting. The researchers say the observations point to enisamium as a viable and accessible option for the treatment of SARS-CoV-2 infection and COVID-19. A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
Vaccines are available, but antivirals are still needed
Since the COVID-19 outbreak first began in Wuhan, China, late last year (2020), vaccines have been developed to prevent the spread of SARS-CoV-2, and several antiviral agents such as remdesivir have been clinically approved for emergency use in COVID-19 cases. However, additional strategies are needed because vaccine roll-out is a slow process and the current antivirals can only be delivered intravenously within the hospital setting.
SARS-CoV-2 RNA polymerase transcribes the viral genome
Within the viral genome of SARS-CoV-2, two open reading frames (ORFs) – 1a and 1b – encode two large polyproteins that are proteolytically cleaved to produce 16 non-structural proteins (nsps). One of these proteins – nsp12 – is the RNA-dependent RNA polymerase that copies and transcribes the SARS-CoV-2 genome. Nsp12 requires nsp7 and nsp8 to perform this process in vitro, but te Velthuis and colleagues say Nsp12 likely requires other nsps such as nsp9 and nsp13, for in vivo processing. Cryogenic electron microscopy structures of nsp12/7/8 and nsp8/9/12/13 complexes from SARS-CoV-2 have already been determined, says the team. Furthermore, the antiviral remdesivir has previously been shown to inhibit the nsp12/7/8 complex and other small molecule inhibitors have been suggested as therapeutic candidates.
Where does enisamium come in?
One drug that has been highlighted by the World Health Organization as a potential candidate for treating SARS-CoV-2 infection is enisamium. This drug is an active inhibitor of influenza A and B viruses that have been licensed for use against influenza in 11 countries of the Commonwealth of Independent States. Research has recently shown that an enisamium metabolite called VR17-04 inhibits activity of the influenza virus RNA polymerase, reduces viral shedding and improves recovery among infected patients.
What did the researchers do?
The researchers showed that enisamium could inhibit the growth of SARS-CoV-2 in normal human bronchial epithelial cells (NHBE) cells and in a human colon epithelial cancer cell line called Caco-2. They also conducted an in vitro assay showing that the metabolite VR17-04 directly inhibits the RNA synthesis activity of the SARS-CoV-2 nsp12/7/8 complex. To confirm the anti-SARS-CoV-2 activity of enisamium, the team conducted a double-blind, randomized, placebo-controlled trial of 373 hospitalized COVID-19 patients who needed medical care either with or without supplementary oxygen. Participants received either enisamium (500 mg per dose) or placebo over the course of 7 days. An interim analysis showed that among those who required supplementary oxygen (n=77), enisamium significantly improved the meantime to recovery, compared with placebo (11.1 versus 13.9 days). No significant difference in recovery time was observed for all patients (n=373) or for those who required medical care without oxygen supplementation (n=296)....
Preprint available in bioRxiv (Jan. 12, 2021):



 Your new post is loading...
Your new post is loading...