Your new post is loading...

|
Scooped by
Juan Lama
|
People infected with a variant first identified in South Africa are more likely to die than those infected with other variants. People infected with the Beta coronavirus variant are more likely to need critical care and to die than are people infected with other variants1. The Beta variant, also known as B.1.351, was first identified in late 2020 in South Africa, where it sparked a second COVID-19 wave before spreading globally. Some evidence has suggested that severe cases of COVID-19 were more common during South Africa’s Beta-driven second wave than during its first wave, caused by the ancestral version of SARS-CoV-2. To determine whether the Beta variant is linked to increased severity, Laith Jamal Abu-Raddad, an infectious-disease epidemiologist at Weill Cornell Medicine—Qatar in Doha, studied infected people in Qatar in early 2021. During that period two variants were circulating: Beta and Alpha, which originated in the United Kingdom in 2020 and is also known as B.1.1.7. The team did not compare Beta with the Delta variant, which is now ripping through much of the world and which has also been linked to heightened severity. People infected with Beta were 25% more likely than those infected with Alpha to develop severe disease , and around 50% more likely to require critical care, as well as 57% more likely to die. This fits with observations at the time, says Abu-Raddad. As Beta surged in Qatar, acute-care admissions doubled, and ICU admissions and deaths quadrupled. “It was very clear we were talking about a more severe variant,” he adds. The findings have not yet been peer reviewed. The study was small, but it is important because its conclusions stem from a careful comparison of the outcomes of infections with different variants in people with similar characteristics, such as age and sex, says Waasila Jassat, a public health medicine specialist at the National Institute for Communicable Diseases in Johannesburg, South Africa. She led a study2 published in July that found that people were around 30% more likely to die after hospitalization during South Africa’s second wave than in its first. Pinning down Beta’s severity will help researchers to anticipate its effects on health-care systems, Jassat adds. As the more-transmissible Delta variant spreads, Beta is now fading in many places where it was once dominant, including South Africa and Qatar. But Abu-Raddad notes that Beta seems to be more resistant to immunity generated by vaccines and previous infections than are other variants, including Delta, and it could begin wreaking havoc again. “We should never underestimate this pathogen.” Research Cited Available as Preprint in medRxiv (August 4, 2021): https://doi.org/10.1101/2021.08.02.21261465

|
Scooped by
Juan Lama
|
Researchers in Belgium report on the case of a 90-year-old woman who was simultaneously infected with two different variants of concern (VOCs) of COVID-19, in a Case Report being presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) held online this year. On March 3 2021, the woman, whose medical history was unremarkable, was admitted to the OLV Hospital in the Belgian city of Aalst after a spate of falls. She tested positive for COVID-19 on the same day. She lived alone and received nursing care at home, and had not been vaccinated against COVID-19. Initially, there were no signs of respiratory distress and the patient had good oxygen saturation. However, she developed rapidly worsening respiratory symptoms, and died five days later. When the patient's respiratory sample was tested for VOCs with PCR, they discovered that she had been infected by two different strains of the virus—one which originated in the UK, known as B.1.1.7 (Alpha), and another that was first detected in South Africa (B.1.351; Beta). The presence of both strains was confirmed by PCR on a second respiratory sample, by sequencing of the S-gene and by whole genome sequencing. "This is one of the first documented cases of co-infection with two SARS-CoV-2 variants of concern", says lead author and molecular biologist Dr. Anne Vankeerberghen from the OLV Hospital in Aalst, Belgium. "Both these variants were circulating in Belgium at the time, so it is likely that the lady was co-infected with different viruses from two different people. Unfortunately, we don't know how she became infected." On December 14, 2020, the UK authorities informed WHO that a variant (B.1.1.7; Alpha) had been detected in the south east of England (Kent). Within a few weeks, this variant took over from the viral strains circulating in this region, and has since spread to more than 50 countries, including Belgium. On December 18, 2020, the South African authorities reported that a variant (B.1.351; Beta) had been detected and was spreading rapidly throughout three provinces of South Africa, and has now been identified in at least 40 countries, including Belgium. In January 2021, scientists in Brazil reported that two people had been simultaneously infected with two different strains of the coronavirus—the Brazilian variant known as B.1.1.28 (E484K) and a novel variant VUI-NP13L, which had previously been discovered in Rio Grande do Sul. But the study has yet to be published in a scientific journal [1]. Previous research has reported people infected with different influenza strains [2]. "Whether the co-infection of the two variants of concern played a role in the fast deterioration of the patient is difficult to say", says Vankeerberghen. "Up to now, there have been no other published cases. However, the global occurrence of this phenomenon is probably underestimated due to limited testing for variants of concern and the lack of a simple way to identify co-infections with whole genome sequencing." She continues, "Since co-infections with variants of concern can only be detected by VOC-analysis of positive samples, we would encourage scientists to perform fast, easy and cheap VOC-analysis by PCR on a large proportion of their positive samples, rather than just whole genome sequencing on a small proportion. Independent of the technique used, being alert to co-infections remains crucial." See also abstract of the research cited (July 10, 2021): https://www.olvz.be/sites/default/files/2021-07/04978-90_year_old_double_infection.pdf

|
Scooped by
Juan Lama
|
GAITHERSBURG, Md., June 11, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company developing next-generation vaccines for serious infectious diseases, today announced preclinical and clinical data on the company's original recombinant protein COVID-19 vaccine candidate, NVX-CoV2373, and for a new vaccine directed against the SARS-CoV-2 Beta (B.1.351) variant, which was originally identified in South Africa. The data show that the vaccines demonstrated strong immunogenicity and protection against both the Alpha (B.1.1.7) variant, which was originally identified in the United Kingdom, and the Beta (B.1.351) variant as well as the original SARS-CoV-2 in animal and human studies. A preprint of the manuscript, 'Immunogenicity and In vivo protection of a variant nanoparticle vaccine that confers broad protection against emerging SARS-CoV-2 variants,' is available at bioRxiv.org and has been submitted for peer review. "While current vaccines are effective against selected SARS-CoV-2 variant strains, newly emerging variants are also being identified that have the ability to overcome vaccine induced immunity," said Matthew Frieman, PhD, Associate Professor of Microbiology and Immunology at the University of Maryland School of Medicine, who collaborated on these studies. "This work demonstrates that variant vaccines that protect against these newly emerging variants have the potential to be highly effective and may produce broader protection against variants we know of and those that will arise in the future. Clinical trials will provide further evidence on the effectiveness of variant vaccines." The studies compared the Beta (B.1.351)-directed vaccine to Novavax' prototype vaccine candidate as standalone, in combination, and as heterologous prime boost vaccine. The findings show a broad array of cellular and humoral responses in animal models against all virus strains evaluated. The Alpha (B.1.1.7) and Beta (B.1.351) variant strains have created public health concerns due to increased transmission rates and lower efficacy of current vaccines seen against Beta (B.1.351). "These data suggest that not only could one booster dose of this variant-directed vaccine potentially provide a robust, protective immune boost after vaccination against the original SARS-CoV-2 virus, but also the potential to provide broad protection against various virus strains if used as a primary vaccine regimen," said Gregory M. Glenn, M.D., President of Research and Development, Novavax. "This broad immune coverage is vital to controlling the pandemic as variants of concern continue to emerge worldwide that could jeopardize the protection created through ongoing COVID-19 vaccination efforts." Study 1: Immunogenicity and Protection in Mice Mice were immunized with NVX-CoV2373 or rS-B.1.351 alone, in combination, or as a heterologous prime boost. Mice vaccinated with any of the four regimens displayed elevated antibody titers against both the original and Beta (B.1.351) spike. Heterologous or bivalent vaccination is highly immunogenic against the prototype spike compared to monovalent approaches, while immunization with monovalent rS-B.1.351 or bivalent spike resulted in the highest anti-B.1.351 spike IgG titers among regimens tested. Additionally, mice immunized with rS-B.1.351 alone produced elevated neutralizing antibody titers to the Alpha (B.1.1.7) and Beta (B.1.351) strains compared to titers upon immunization with the prototype-directed strain. Titers were similar in the heterologous and bivalent vaccine groups. Antibodies produced after vaccination with rS-B.1.351 inhibited binding between human angiotensin converting enzyme-2 receptor (hACE2) and the variant spike or original spike to the same degree. This indicates that rS-B.1.351 can efficiently protect "backward" against original SARS-CoV-2 strains. In contrast, NVX-CoV2373 was less efficient at protecting "forward" against the Beta (B.1.351) variant strain. Whether immunized with prototype vaccine or rS-B.1.351 alone, in combination, or as a heterologous prime boost, mice were protected when challenged with live Alpha (B.1.1.7) or Beta (B.1.351) variant strains of SARS-CoV-2. Study 2: Anamnestic Response in Baboons A cohort of baboons that were originally immunized with prototype vaccine were boosted approximately one year later with one or two doses of rS-B.1.351. Seven days after the first rS-B.1.351 boost, those that originally received adjuvanted prototype-directed vaccine exhibited a strong immune response, with anti-Spike IgG titers that were higher than the peak immune response observed during the primary immunization series. The rS-B.1.351 boost elicited comparable antibody titers against original and rS-B.1.351 spike. Further, the same cohort exhibited a strong neutralizing response to Alpha (B.1.1.7) and Beta (B.1.351) strains and hACE2-inhibiting antibody response following the boost, despite having undetectable titers before the boost. High neutralizing antibody titers were observed seven days post-boost, demonstrating "a robust, durable antibody response even one year after the primary vaccination series." These results suggest that one dose of a variant-directed vaccine may be sufficient for boosting regimens after previous immunization with a COVID-19 vaccine based on the original spike strain. Study 3: Robust Antibody Response in Humans NVX-CoV2373 is currently being studied in multiple clinical trials, including in locations where Alpha (B.1.1.7) and Beta (B.1.351) variant strains are widespread. Thirty randomly selected human serum samples from Phase 2 clinical trial participants after their second dose of the vaccine were assayed. The sera were analyzed for their ability to neutralize Alpha (B.1.1.7) and Beta (B.1.351) strains. The trial participants' sera demonstrated a neutralizing capacity of the Alpha (B.1.1.7) strain equal to NVX-CoV2373, with a modest reduction in neutralizing capacity against the Beta (B.1.351) strain. These data support the development and production of a Beta (B.1.351) targeted vaccine, which also demonstrated to be efficient at protecting mice against Beta (B.1.351) and to induce a strong response in primates originally immunized with NVX-CoV2373. Furthermore, the data support that a booster vaccine containing a variant strain could both increase antibody levels as well as broaden coverage. Novavax expects to initiate further clinical testing of rS-B.1.351 in the fall of 2021.... See bioRxiv (June 9, 2021): https://doi.org/10.1101/2021.06.08.447631

|
Scooped by
Juan Lama
|
BACKGROUND The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants threatens progress toward control of the coronavirus disease 2019 (Covid-19) pandemic. In a phase 1–2 trial involving healthy adults, the NVX-CoV2373 nanoparticle vaccine had an acceptable safety profile and was associated with strong neutralizing-antibody and antigen-specific polyfunctional CD4+ T-cell responses. Evaluation of vaccine efficacy was needed in a setting of ongoing SARS-CoV-2 transmission. METHODS In this phase 2a–b trial in South Africa, we randomly assigned human immunodeficiency virus (HIV)–negative adults between the ages of 18 and 84 years or medically stable HIV-positive participants between the ages of 18 and 64 years in a 1:1 ratio to receive two doses of either the NVX-CoV2373 vaccine (5 μg of recombinant spike protein with 50 μg of Matrix-M1 adjuvant) or placebo. The primary end points were safety and vaccine efficacy against laboratory-confirmed symptomatic Covid-19 at 7 days or more after the second dose among participants without previous SARS-CoV-2 infection. RESULTS Of 6324 participants who underwent screening, 4387 received at least one injection of vaccine or placebo. Approximately 30% of the participants were seropositive for SARS-CoV-2 at baseline. Among 2684 baseline seronegative participants (94% HIV-negative and 6% HIV-positive), predominantly mild-to-moderate Covid-19 developed in 15 participants in the vaccine group and in 29 in the placebo group (vaccine efficacy, 49.4%; 95% confidence interval [CI], 6.1 to 72.8). Vaccine efficacy among HIV-negative participants was 60.1% (95% CI, 19.9 to 80.1). Of 41 sequenced isolates, 38 (92.7%) were the B.1.351 variant. Post hoc vaccine efficacy against B.1.351 was 51.0% (95% CI, −0.6 to 76.2) among the HIV-negative participants. Preliminary local and systemic reactogenicity events were more common in the vaccine group; serious adverse events were rare in both groups. CONCLUSIONS The NVX-CoV2373 vaccine was efficacious in preventing Covid-19, with higher vaccine efficacy observed among HIV-negative participants. Most infections were caused by the B.1.351 variant. Published in NEJM (May 5, 2021): https://doi.org/10.1056/NEJMoa2103055

|
Scooped by
Juan Lama
|
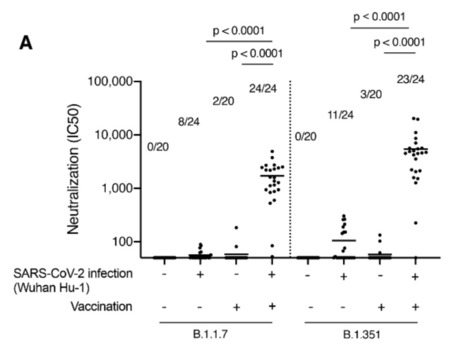
SARS-CoV-2 vaccine rollout has coincided with the spread of variants of concern. We investigated if single dose vaccination, with or without prior infection, confers cross protective immunity to variants. We analyzed T and B cell responses after first dose vaccination with the Pfizer/BioNTech mRNA vaccine BNT162b2 in healthcare workers (HCW) followed longitudinally, with or without prior Wuhan-Hu-1 SARS-CoV-2 infection. After one dose, individuals with prior infection showed enhanced T cell immunity, antibody secreting memory B cell response to spike and neutralizing antibodies effective against B.1.1.7 and B.1.351. By comparison, HCW receiving one vaccine dose without prior infection showed reduced immunity against variants. B.1.1.7 and B.1.351 spike mutations resulted in increased, abrogated or unchanged T cell responses depending on human leukocyte antigen (HLA) polymorphisms. Single dose vaccination with BNT162b2 in the context of prior infection with a heterologous variant substantially enhances neutralizing antibody responses against variants. Published in Science (April 30, 2021): https://doi.org/10.1126/science.abh1282

|
Scooped by
Juan Lama
|
The coronavirus variant discovered in South Africa can "break through" Pfizer/BioNTech's COVID-19 vaccine to some extent, a real-world data study in Israel found, though its prevalence in the country is low and the research has not been peer reviewed. The study, released on Saturday, compared almost 400 people who had tested positive for COVID-19, 14 days or more after they received one or two doses of the vaccine, against the same number of unvaccinated patients with the disease. It matched age and gender, among other characteristics. The South African variant, B.1.351, was found to make up about 1% of all the COVID-19 cases across all the people studied, according to the study by Tel Aviv University and Israel’s largest healthcare provider, Clalit. But among patients who had received two doses of the vaccine, the variant’s prevalence rate was eight times higher than those unvaccinated - 5.4% versus 0.7%. This suggests the vaccine is less effective against the South African variant, compared with the original coronavirus and a variant first identified in Britain that has come to comprise nearly all COVID-19 cases in Israel, the researchers said. “We found a disproportionately higher rate of the South African variant among people vaccinated with a second dose, compared to the unvaccinated group. This means that the South African variant is able, to some extent, to break through the vaccine’s protection,” said Tel Aviv University’s Adi Stern. The researchers cautioned, though, that the study only had a small sample size of people infected with the South African variant because of its rarity in Israel. They also said the research was not intended to deduce overall vaccine effectiveness against any variant, since it only looked at people who had already tested positive for COVID-19, not at overall infection rates. Pfizer and BioNTech could not be immediately reached for comment outside business hours. The companies said on April 1 that their vaccine was around 91% effective at preventing COVID-19, citing updated trial data that included participants inoculated for up to six months. In respect to the South African variant, they said that among a group of 800 study volunteers in South Africa, where B.1.351 is widespread, there were nine cases of COVID-19, all of which occurred among participants who got the placebo. Of those nine cases, six were among individuals infected with the South African variant. Some previous studies have indicated that the Pfizer/BioNTech shot was less potent against the B.1.351 variant than against other variants of the coronavirus, but still offered a robust defence. While the results of the study may cause concern, the low prevalence of the South African strain was encouraging, according to Stern. “Even if the South African variant does break through the vaccine’s protection, it has not spread widely through the population,” said Stern, adding that the British variant may be “blocking” the spread of the South African strain. Almost 53% of Israel’s 9.3 million population has received both Pfizer doses. Israel has largely reopened its economy in recent weeks while the pandemic appears to be receding, with infection rates, severe illness and hospitalizations dropping sharply. About a third of Israelis are below the age of 16, which means they are still not eligible for the shot. Findings available in medRxiv (April 9, 2021): https://doi.org/10.1101/2021.04.06.21254882

|
Scooped by
Juan Lama
|
The NIH on Wednesday announced the start of a phase 1 clinical trial evaluating Moderna’s variant-specific COVID-19 vaccine, saying the shot has been administered to trial participants. Moderna shipped doses of the vaccine — a modified version of its mRNA-1273 vaccine, which has been authorized for use in the U.S. since December — to the NIH last month for testing. The vaccine was tweaked to target the B.1.351 variant, which was first identified in South Africa. According to the NIH, the new trial will enroll 210 healthy adults at four clinical research sites in Atlanta, Cincinnati, Nashville and Seattle. Investigators at the four sites anticipate that the trial will be fully enrolled by the end of April, the NIH said. The modified vaccine will be studied in both vaccinated and unvaccinated adults. The vaccinated adults already received mRNA-1273 last year as part of a clinical trial. They will be randomly assigned to receive either a single booster vaccination of 50 µg of the modified vaccine, mRNA-1273.351, or a single vaccination containing one 25 µg dose of mRNA-1273 and one 25 µg dose of mRNA-1273.351. A separate clinical trial will assess a booster shot of the original vaccine in the remaining vaccinated study participants. According to National Institute of Allergy and Infectious Diseases Director Anthony S. Fauci, MD, the B.1.351 variant has been detected in at least nine U.S. states. The NIAID, which co-developed the vaccine with Moderna, is leading and funding the new trial. “Preliminary data show that the COVID-19 vaccines currently available in the United States should provide an adequate degree of protection against SARS-CoV-2 variants,” Fauci said in the NIH release. “However, out of an abundance of caution, NIAID has continued its partnership with Moderna to evaluate this variant vaccine candidate should there be a need for an updated vaccine.” NIH Press Release (March 31, 2021): https://www.nih.gov/news-events/news-releases/nih-clinical-trial-evaluating-moderna-covid-19-variant-vaccine-begins

|
Scooped by
Juan Lama
|
Pfizer Inc and BioNTech said on Thursday their COVID-19 vaccine is around 91% effective at preventing the disease, citing updated trial data that included participants inoculated for up to six months. The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said.The shot was also 100% effective in preventing illness among trial participants in South Africa, where a new variant called B1351 is dominant, although that rate was derived from a relatively small number of nine infections observed there, which were all in the placebo group, Pfizer said. While the new overall efficacy rate of 91.3% is lower than the 95% originally reported in November for its 44,000-person trial, a number of variants have become more prevalent around the world since then. Pfizer’s Chief Executive Officer Albert Bourla said the updated result, which includes data on more than 12,000 people fully inoculated for at least six months, positions the drugmakers to submit for full U.S. regulatory approval. The vaccine is currently authorized on an emergency basis by the U.S. Food and Drug Administration. The trial data “provide the first clinical results that a vaccine can effectively protect against currently circulating variants, a critical factor to reach herd immunity and end this pandemic for the global population,” Ugur Sahin, chief executive officer at BioNTech, said in a statement. Experts fear new variants of COVID-19 from South Africa and Brazil may be resistant to existing vaccines and treatment. More than 300 cases of the South African variant have been detected in more than 25 U.S. states and jurisdictions, according to federal data. Lab tests have previously indicated that BioNTech’s vaccine was less potent but still offered a robust defense against the B1351 variant that first emerged in South Africa. Still, BioNTech reiterated this week there would likely be a future need for booster shots that specifically address new variants and that the group was preparing to upgrade its vaccine when needed. BioNTech has said that it started testing a modified vaccine version against the South African mutant in March for early indications on safety and efficacy but a product for later market launch would require yet another redesign and more tests. The updated trial data would not prompt the company to change that development strategy, a BioNTech spokeswoman said. Pfizer's Press Release (April 1, 2021): https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-confirm-high-efficacy-and-no-serious

|
Scooped by
Juan Lama
|
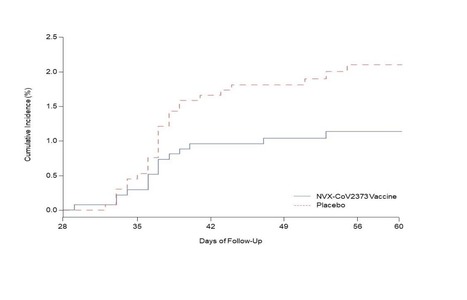
BACKGROUND Assessment of the safety and efficacy of vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in different populations is essential, as is investigation of the efficacy of the vaccines against emerging SARS-CoV-2 variants of concern, including the B.1.351 (501Y.V2) variant first identified in South Africa. METHODS We conducted a multicenter, double-blind, randomized, controlled trial to assess the safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) in people not infected with the human immunodeficiency virus (HIV) in South Africa. Participants 18 to less than 65 years of age were assigned in a 1:1 ratio to receive two doses of vaccine containing 5×1010 viral particles or placebo (0.9% sodium chloride solution) 21 to 35 days apart. Serum samples obtained from 25 participants after the second dose were tested by pseudovirus and live-virus neutralization assays against the original D614G virus and the B.1.351 variant. The primary end points were safety and efficacy of the vaccine against laboratory-confirmed symptomatic coronavirus 2019 illness (Covid-19) more than 14 days after the second dose. RESULTS Between June 24 and November 9, 2020, we enrolled 2026 HIV-negative adults (median age, 30 years); 1010 and 1011 participants received at least one dose of placebo or vaccine, respectively. Both the pseudovirus and the live-virus neutralization assays showed greater resistance to the B.1.351 variant in serum samples obtained from vaccine recipients than in samples from placebo recipients. In the primary end-point analysis, mild-to-moderate Covid-19 developed in 23 of 717 placebo recipients (3.2%) and in 19 of 750 vaccine recipients (2.5%), for an efficacy of 21.9% (95% confidence interval [CI], −49.9 to 59.8). Among the 42 participants with Covid-19, 39 cases (92.9%) were caused by the B.1.351 variant; vaccine efficacy against this variant, analyzed as a secondary end point, was 10.4% (95% CI, −76.8 to 54.8). The incidence of serious adverse events was balanced between the vaccine and placebo groups. CONCLUSIONS A two-dose regimen of the ChAdOx1 nCoV-19 vaccine did not show protection against mild-to-moderate Covid-19 due to the B.1.351 variant. Published in NEJM (March 16, 2021): https://doi.org/10.1056/NEJMoa2102214

|
Scooped by
Juan Lama
|
Background The emergence of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) variants threatens progress toward control of the Covid-19 pandemic. Evaluation of Covid-19 vaccine efficacy against SARS-CoV-2 variants is urgently needed to inform vaccine development and use. Methods In this phase 2a/b, multicenter, randomized, observer-blinded, placebo-controlled trial in South Africa, healthy human immunodeficiency virus (HIV)-negative adults (18 to 84 years) or medically stable people living with HIV (PLWH) (18 to 84 years) were randomized in a 1:1 ratio to receive two doses, administered 21 days apart, of either NVX-CoV2373 nanoparticle vaccine (5 micrograms recombinant spike protein with 50 micrograms Matrix-M1 adjuvant) or placebo. The primary endpoints were safety and vaccine efficacy greater than or equal to 7 days following the second dose against laboratory-confirmed symptomatic Covid-19 in previously SARS-CoV-2 uninfected participants. Results A total of 4387 participants were randomized and dosed at least once, 2199 with NVX CoV2373 and 2188 with placebo. Approximately 30% of participants were seropositive at baseline. Among 2684 baseline seronegative participants (94% HIV negative; 6% PLWH), there were 15 and 29 predominantly mild to moderate Covid-19 cases in NVX CoV2373 and placebo recipients, respectively; vaccine efficacy was 49.4% (95% confidence interval [CI]: 6.1 to 72.8). Efficacy in HIV negative participants was 60.1% (95% CI: 19.9 to 80.1), and did not differ by baseline serostatus. Of the primary endpoint cases with available whole genome sequencing, 38 (92.7%) of 41 were the B.1.351 variant. Post-hoc vaccine efficacy against B.1.351 was 51.0% (95% CI: -0.6 to 76.2) in HIV-negative participants. Among placebo recipients, the incidence of symptomatic Covid-19 was similar in baseline seronegative vs baseline seropositive participants during the first 2 months of follow-up (5.3% vs 5.2%). Preliminary local and systemic reactogenicity were primarily mild to moderate and transient, and higher with NVX CoV2373; serious adverse events were rare in both groups. Conclusions The NVX-CoV2373 vaccine was efficacious in preventing Covid-19, which was predominantly mild to moderate and due to the B.1.351 variant, while evidence of prior infection with the presumptive original SARS CoV-2 did not confer protection against probable B.1.351 disease. (Funded by Novavax, The Bill and Melinda Gates Foundation, and the Coalition for Epidemic Preparedness Innovations; ClinicalTrials.gov number, NCT04533399) Preprint available in medRxiv (March 3, 2021): https://doi.org/10.1101/2021.02.25.21252477

|
Scooped by
Juan Lama
|
Small efficacy trial doesn’t show whether vaccine may prevent severe disease caused by antibody-dodging strain. Another COVID-19 vaccine has run into trouble in South Africa, showing less protection there than elsewhere because a SARS-CoV-2 variant that can apparently dodge key antibodies has become widespread. In the wake of the new finding, the country, which had pinned its pandemic hopes on this particular vaccine, halted an immunization campaign launched only last week. The stakes are high for the vaccine globally because its makers, AstraZeneca and the University of Oxford, hope it will be widely used in developing countries; they project they can produce 3 billion doses this year for about $3 each, far more product at a far lower price than any other vaccine shown to offer protection against COVID-19. Yet the South African trial of the vaccine, conducted in about 2000 people, found such a low efficacy against mild and moderate disease, under 25%, that it would not meet minimal international standards for emergency use. But scientists are hopeful it might still prevent severe disease and death—arguably the most important job for any COVID-19 vaccine. That was impossible to tell from this placebo-controlled trial because it was small and recruited relatively healthy, young people—their average age was only 31. None of the subjects in either arm of the study developed severe disease or required hospitalization. The new results are a “reality check,” Shabir Madhi of the University of the Witwatersrand, the trial’s principal investigator, said today at a press conference. “It is time, unfortunately, for us to recalibrate our expectations of COVID-19 vaccines, as well as how we go about deciding how to respond to the COVID-19 pandemic in South Africa as well as globally.” COVID-19 vaccines made by Johnson & Johnson (J&J) and Novavax have also been shown to offer weaker protection against B.1.351 (also known as 501.V2), the SARS-CoV-2 variant that now causes the vast majority of all infections in South Africa, than against older variants. The vaccines’ efficacy against mild disease in South Africa was 57% for J&J and 49% for Novavax—lower than in any other country they were tested. But the J&J vaccine, which was put to the test in the largest of the studies, convincingly protected against severe disease and death, even against the B.1.351 variant, and Madhi remains “somewhat optimistic” that the AstraZeneca-Oxford vaccine will, too; the results are not “all doom and gloom,” he said. SARS-CoV-2–targeting antibodies triggered by the J&J vaccine were “very similar,” he said, to those elicited by the AstraZeneca-Oxford candidate, and the two vaccines are based on a similar technology: Both induce the body to make the spike surface protein of SARS-CoV-2 by delivering its genes in a harmless adenovirus. In a 44,000-person trial, the J&J vaccine prevented 85% of severe cases and completely protected people from hospitalization and death in several countries, including the 15% of the participants who were from South Africa. Madhi and the research team had planned to report the results tomorrow, but the Financial Times ran a story on Saturday based on a leaked copy of the findings. Madhi and his co-workers have submitted a paper describing their data to a preprint server and expect it will post tomorrow The AstraZeneca-Oxford vaccine has produced confusing results from the start. Earlier preliminary results from trials in different countries showed a wide range of success rates against mild and moderate disease, but researchers have had difficulty interpreting the data because of differences in dose, intervals between doses, and variants in circulation. Just on Friday, a study suggested the vaccine offered strong protection against a more transmissible variant, B.1.1.7, that exploded in the United Kingdom and is now spreading fast throughout Europe. In South Africa, the vaccine was given in two doses spaced 21 to 35 days apart. Antibodies made by vaccine recipients can typically “neutralize” SARS-CoV-2, meaning they can prevent it from infecting cells in culture experiments. But lab studies show they have far less power against B.1.351. Madhi stressed that the vaccine may still trigger a powerful T cell response, which can target and eliminate cells the variant manages to infect. He presented a test tube study showing how the mutations in the spike protein that allow B.1.351 to dodge neutralizing antibodies have little impact on T cell responses. “We believe that those T cell responses will still remain intact despite the mutations that exists in a B.1.351 variant,” Madhi said. The AstraZeneca-Oxford vaccine trial, which ran from June to November 2020, found that starting 2 weeks after the second dose—when participants presumably were fully immunized—19 cases of mild or moderate disease developed among the vaccinated, versus 23 in the placebo group, resulting in an efficacy of 21.9%. That is far below the 50% minimum required for emergency use authorization in many countries. Researchers sequenced the viruses that infected trial participants and found a strong link between vaccine failure and B.1.351’s explosion in South Africa. In people who received one dose of the vaccine before the variant began to spread widely, efficacy against mild and moderate disease was still a respectable 75%. South Africa last week received 1 million doses of the AstraZeneca-Oxford vaccine and began to offer them to health care workers, making it the first COVID-19 vaccine available in the country outside of clinical trials. Epidemiologist Salim Abdool Karim, who co-chairs the South African Ministerial Advisory Committee on COVID-19, said at the press conference that the rollout of the vaccine in South Africa “needs to be put on temporary hold” in light of the disappointing results. Barry Schoub, who leads a government advisory subcommittee on COVID-19 vaccines, says, “We may need to look at combinations of the [AstraZeneca-Oxford] vaccine with other vaccines, which may in fact synergistically give a very good response.” The Oxford team that originally designed the vaccine says it has already begun to work on a second-generation candidate that targets the mutated spike protein of the B.1.351 variant. Oxford’s Sarah Gilbert, who leads that effort, suggested in a press statement that a reformulated vaccine might be given as a booster shot to the existing one. “This is the same issue faced by all of the vaccine developers, and we will continue to monitor the emergence of new variants that arise in readiness for a future strain change,” Gilbert noted.

|
Scooped by
Juan Lama
|
Current vaccines likely still work, scientists say, but their power may be declining. News from U.S. manufacturer Moderna that its COVID-19 vaccine is still “expected to be protective” against a virus variant first detected in South Africa came as a relief to scientists and the public. But the 25 January announcement included a caveat: Antibodies triggered by the vaccine appear to be a little less potent against the new variant, named B.1.351, than the one the vaccine was developed for. So researchers were perhaps even more relieved to hear the company will start development of booster shots tailored to B.1.351 and other variants. “These are exactly the steps that I hoped to see,” says virologist Trevor Bedford of the Fred Hutchinson Cancer Research Center. “It may well not be necessary to have a vaccine update in the fall, but taking these steps now is the right course of action.” Other vaccinemakers are also contemplating updates. Scientists have grown increasingly concerned that new coronavirus variants may worsen the pandemic. B.1.1.7, first detected in England and now spreading globally, has been shown to be more transmissible; on 22 January, the U.K. government said it may be deadlier as well. B.1.351 and a very similar variant named P.1 that originated in Brazil’s Amazonas state are suspected of evading immunity in people who were vaccinated or previously infected. Now, researchers from Moderna and the Vaccine Research Center at the U.S. National Institutes of Health have tested the potency of antibodies from eight people who had received the company’s vaccine against a retrovirus modified to express the mutated spike proteins of B.1.351 and B.1.1.7. In a preprint, they report that antibodies neutralized the virus in both cases. But for B.1.351, the levels needed were six times higher than for virus expressing the original protein. A similar study by virologist David Ho of Columbia University, under review at Nature and posted as a preprint on bioRxiv, found that the serum of 22 people vaccinated with Moderna’s vaccine or a similar one from Pfizer was six to nine times less potent against B.1.351, and serum from 20 previously infected people was 11 to 33 times less potent. Researchers in South Africa, meanwhile, have found that antibodies from six recovered patients were six to 200 times less effective at neutralizing B.1.351. Such drops sound alarming, but the vaccines produced by Pfizer and Moderna trigger very high levels of antibodies, which likely compensates for the decline in potency, says Florian Krammer, a vaccine researcher at the Icahn School of Medicine at Mount Sinai. Besides, antibodies are only one part of the immune response; the vaccines also trigger T cells. Krammer is “quite optimistic” that both vaccines will still protect against B.1.351 and P.1. “However, this is worrisome for vaccines that are not as potent in inducing neutralizing antibodies as the two mRNA [messenger RNA] vaccines.” Others agree the results don’t spell doom yet. “Given the high starting point, it’s conceivable [vaccine efficacy] could drop only slightly,” Bedford says. Immunity is not binary, adds Jeremy Farrar, head of the Wellcome Trust: “It doesn’t suddenly turn on and turn off.” A drop in antibody potency could have more subtle effects, such as immunity waning a bit faster, he says. The results with sera from recovered patients also suggest the risk of reinfection with COVID-19 may be rising, especially for people who produced low levels of antibodies during their first encounter with the virus, says Stephen Goldstein, a virologist at the University of Utah. “Most of these people I expect to still have good protection from serious disease. It’s on a spectrum, though.” Moderna says it will start phase I trials of two booster strategies: a third dose of its current vaccine, or of a slightly different one in which the mRNA has been tweaked to incorporate B.1.351’s mutations. They may be given to volunteers 6 to 12 months after the initial immunization, Moderna Chief Medical Officer Tal Zaks said in a call with investors. Pfizer, in an email to Science, wrote that it, too, is “laying the groundwork to respond quickly if a future variant of SARS-CoV-2 is unresponsive to existing vaccines.” Novavax, which is in late-stage trials with a vaccine based the spike protein, says it is “testing sera against the new strains.” Georgetown University virologist Angela Rasmussen says it’s “very wise” to start to prepare boosters now. “It’s also wise to begin thinking about how they will be distributed,” she adds. “For example, will they be allocated to regions with evidence that B.1.351 is circulating?” Regulators still need to spell out what trials they would require for updated vaccines. At a press conference on Monday, World Health Organization official Bruce Aylward said work to define a regulatory pathway was “kicking off right now.” Scientists also need to agree on faster ways to address any concerns about immune escape variants, says Farrar, and standardize the way they test antibodies’ potency: “We need harmonization of the assays, so we can compare the results and it doesn’t matter which lab you’re in.” Animal experiments need to be coordinated as well. Vincent Munster, a virologist at the U.S. National Institute of Allergy and Infectious Diseases, says he has already vaccinated hamsters and will challenge them with virus variants in the next couple of weeks. “These studies take a lot of coordination and we are discussing the need for a more planned approach to prepare for other novel variants emerging,” Munster says. The most timely answers on B.1.351 may come from humans, however. Efficacy trials of several vaccines, including the Pfizer one, are ongoing in South Africa; Tulio de Oliveira, a virologist at the University of KwaZulu-Natal, says researchers are now sequencing the virus from 150 study participants who became infected. “We’re going have the results in 36 hours,” he says. But only after the trial is unblinded next week will researchers know how many of these infections occurred in people who received the vaccine instead of a placebo. Ho’s paper also sheds some light on how B.1.351 escapes the immune response. The team produced retroviruses with spike proteins incorporating each of B.1.351’s nine mutations separately, as well as all at once. A mutation named E484K accounted for much of the effect, they found. “E484K is really the bad boy here,” Goldstein says. Brazil’s P.1 variant has the same mutation, which might be a sign that the virus has few other tricks to evade immunity, he says: “The virus has a lot of room to evolve but not infinite room. We may have come upon one of the worst possible mutations already.” But other researchers say the plethora of recent changes is a warning sign that the coronavirus may have more surprises in store—and that the world needs to administer existing vaccines as fast as possible. “I think we need to stop the virus from replicating however we can,” Ho says. “Otherwise, it will keep accumulating more mutations.”

|
Scooped by
Juan Lama
|
Moderna is studying whether booster doses could be necessary after finding its vaccine was less effective against one coronavirus variant. Moderna is studying adding booster doses to its vaccine regimen after finding its Covid-19 vaccine was less potent against a coronavirus variant that was first identified in South Africa, the company said Monday. In lab research that involved testing whether blood from people who had received the vaccine could still fend off different coronavirus variants, scientists found that there was a sixfold reduction in the vaccine’s neutralizing power against the variant, called B.1.351, than against earlier forms of the coronavirus, Moderna reported. There was no loss in neutralization levels against a different variant, called B.1.1.7, that was first identified in the United Kingdom. Both variants are thought to be more transmissible than other forms of the SARS-CoV-2 virus. Moderna said that despite the reduction in neutralizing antibodies against B.1.351, the antibody levels generated by its vaccine “remain above levels that are expected to be protective.” Still, it said it was going to start testing whether adding a booster dose to its existing two-dose regimen could increase the levels of neutralizing antibodies even further, and that it was going to start investigating a booster specifically designed against B.1.351. “These lower titers [of antibodies against B.1.351] may suggest a potential risk of earlier waning of immunity to the new B.1.351 strains,” Moderna said. The announcement from Moderna gets at a nuance that scientists have been trying to stress as fears around vaccines and variants grew. Both the Moderna vaccine and the immunization from Pfizer-BioNTech produce such powerful levels of immune protection — generating higher levels of antibodies on average than people who recover from a Covid-19 infection have — that they should be able to withstand some drop in their potency without really losing their ability to guard people from getting sick. “There is a very slight, modest diminution in the efficacy of a vaccine against it, but there’s enough cushion with the vaccines that we have that we still consider them to be effective,” Anthony Fauci, the top U.S. infectious diseases official, said Monday on the “Today” show. The coronavirus has been evolving throughout the pandemic, and scientists had expected that eventually, the virus would change so much that vaccines would need to be upgraded to better match dominant variants. But the appearance in recent months of the variants, which picked up mutations at much higher rates than the coronavirus was adding at the beginning of the pandemic, has moved up the date at which that might need to occur. Experts say they need to now figure out how much less effective the vaccines can get before upgrades are needed, and what the regulatory process for approving such tweaks would look like. Research cited available at bioRxiv (Jan. 25, 2021): https://doi.org/10.1101/2021.01.25.427948 Moderna's Press Release: https://investors.modernatx.com/news-releases/news-release-details/moderna-covid-19-vaccine-retains-neutralizing-activity-against
|

|
Scooped by
Juan Lama
|
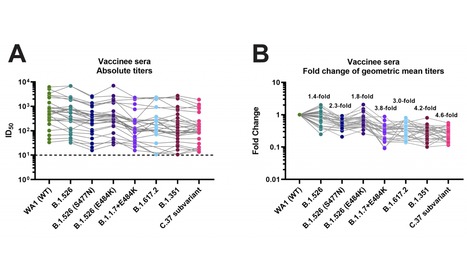
Highly efficacious vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed. However, the emergence of viral variants that are more infectious than the earlier SARS-CoV-2 strains is concerning. Several of these viral variants have the potential to partially escape neutralizing antibody responses warranting continued immune-monitoring. Here, we tested a number of currently circulating viral variants of concern/interest, including B.1.526 (Iota), B.1.1.7+E484K (Alpha), B.1.351 (Beta), B.1.617.2 (Delta) and C.37 (Lambda) in neutralization assays using a panel of post-mRNA vaccination sera. The assays were performed with authentic SARS-CoV-2 clinical isolates in an assay that mimics physiological conditions. We found only small decreases in neutralization against B.1.526 and an intermediate phenotype for B.617.2. The reduction was stronger against a sub-variant of C.37, followed by B.1.351 and B.1.1.7+E484K. C.37 is currently circulating in parts of Latin America and was detected in Germany, the US and Israel. Of note, reduction in a binding assay that also included P.1, B.1.617.1 (Kappa) and A.23.1 was negligible. Taken together, these findings suggest that mRNA SARS-CoV-2 vaccines may remain effective against these viral variants of concern/interest and that spike binding antibody tests likely retain specificity in the face of evolving SARS-CoV-2 diversity. Preprint Available in medRxiV (July 23, 2021): https://doi.org/10.1101/2021.07.21.21260961

|
Scooped by
Juan Lama
|
The BNT162b2 mRNA vaccine is highly effective against SARS-CoV-2. However, apprehension exists that variants of concern (VOCs) may evade vaccine protection, due to evidence of reduced neutralization of the VOCs B.1.1.7 and B.1.351 by vaccine sera in laboratory assays. We performed a matched cohort study to examine the distribution of VOCs in infections of BNT162b2 mRNA vaccinees from Clalit Health Services (Israel) using viral genomic sequencing, and hypothesized that if vaccine effectiveness against a VOC is reduced, its proportion among breakthrough cases would be higher than in unvaccinated controls. Analyzing 813 viral genome sequences from nasopharyngeal swabs, we showed that vaccinees who tested positive at least 7 days after the second dose were disproportionally infected with B.1.351, compared with controls. Those who tested positive between 2 weeks after the first dose and 6 days after the second dose were disproportionally infected by B.1.1.7. These findings suggest reduced vaccine effectiveness against both VOCs within particular time windows. Our results emphasize the importance of rigorously tracking viral variants, and of increasing vaccination to prevent the spread of VOCs. At early time points after vaccination with a single dose or two doses of the BNT162b2 mRNA COVID-19 vaccine, breakthrough SARS-CoV-2 infections can be disproportionately caused by the B.1.1.7 or B.1.351 variants of concern, underlining the need to ensure rapid and complete vaccination. Published in Nature Medicine (June 14, 2021): https://doi.org/10.1038/s41591-021-01413-7

|
Scooped by
Juan Lama
|
The SARS-CoV-2 B.1.617.2 Variant of Concern (VOC), first detected in India, is now dominant in the UK, having rapidly displaced the B.1.1.7 strain that emerged in the UK with the second COVID-19 wave in late 2020. The efficacy of currently licensed COVID-19 vaccines against B.1.617.2 is unknown; although it possesses 12 mutations in its spike protein relative to the wildtype SARS-CoV-2 first detected in Wuhan, China, in December, 2019, B.1.617.2 lacks mutations at amino acid positions 501 or 484 in its ACE2 receptor-binding domain, commonly associated with VOCs (appendix p 2) or escape from neutralising antibodies (NAbs). To determine vaccine-induced NAb escape by B.1.617.2 and compare activity to previous strains with existing estimates for population-based vaccine efficacy, we carried out an initial analysis of the Legacy study, established in January, 2021, by University College London Hospital and the Francis Crick Institute in London, UK, to track serological responses to vaccination in prospectively recruited staff volunteers (appendix p 6). A detailed description of the methods, including the clinical cohort, virus culture conditions, genetic sequencing, and neutralisation assays, and the statistical analysis are available in the appendix (p 8). The Legacy study was approved by London Camden and Kings Cross Health Research Authority Research and Ethics committee (IRAS number 286469) and sponsored by University College London. Published in the Lancet (June 3, 2021):

|
Scooped by
Juan Lama
|
Data from Qatar provide strongest evidence yet that COVID-19 vaccines can stop strains thought to pose a threat to immunization efforts. Qatar’s second wave of COVID-19 was a double whammy. In January, after months of relatively few cases and deaths, the Gulf nation saw a surge driven by the fast-spreading B.1.1.7 variant, which was first identified in the United Kingdom. Weeks later, the B.1.351 strain, which is linked to reinfections and dampened vaccine effectiveness, took hold. Amid this storm, researchers in Qatar have found some of the strongest evidence yet that current vaccines can quell variants such as B.1.351. Clinical trials in South Africa — where B.1.351 was first identified — had suggested that vaccines would take a hit against such variants. But this study offers a fuller picture of what countries battling such variants can expect. People in Qatar who received two doses of the Pfizer–BioNTech vaccine were 75% less likely to develop a case of COVID-19 caused by B.1.351 than were unvaccinated people, and had near-total protection from severe disease caused by that strain. The findings — published on 5 May in The New England Journal of Medicine1 — suggest that current RNA vaccines are a potent weapon against the most worrisome immune-evading variants. Pfizer, based in New York City, and BioNTech, in Mainz, Germany, are developing an updated RNA vaccine targeting B.1.351, as is Moderna, based in Cambridge, Massachusetts. Early results from Moderna’s efforts suggest that a booster shot of the updated vaccine triggers a strong response against B.1.351. “I think this variant is probably the worst of all the variants we know,” says Laith Jamal Abu-Raddad, an infectious-disease epidemiologist at Weill Cornell Medicine—Qatar in Doha, who led the Qatari study. “We have the tools, despite these variants, to control at least the severe forms of infection — and this should work quite well on transmission.” Weaker protection Researchers in South Africa identified B.1.351 in late 2020, and it’s now the predominant strain there. Laboratory studies show that the variant harbours mutations that blunt the effects of virus-blocking antibodies, and trials suggest that some COVID-19 vaccines are significantly less effective against the strain than against others. Early lab research suggested that RNA vaccines, including the Pfizer–BioNTech jab, would be weakened by B.1.351, but probably not fully compromised. In April, the companies announced that a small trial in South Africa had found the vaccine to be fully effective against B.1.351, but the study of 800 people recorded a total of just 6 infections caused by B.1.351 in the placebo group, so efficacy might have been much lower. Abu-Raddad’s team analysed tens of thousands of COVID-19 cases that occurred between the start of Qatar’s vaccination campaign in late December and the end of March. Genome sequencing showed that B.1.1.7 and B.1.351 were the predominant coronavirus lineages during this period and, from mid-February, each accounted for about half of the country’s cases. The researchers compared SARS-CoV-2 infection rates in vaccinated people with those in unvaccinated controls. People who received two vaccine doses were about 90% less likely to develop an infection caused by B.1.1.7, echoing findings from Israel, the United Kingdom and elsewhere. The researchers identified around 1,500 ‘breakthrough’ infections caused by the B.1.351 variant in vaccinated individuals, but only 179 of these occurred more than 2 weeks after the second dose. There were hardly any severe cases of COVID-19 caused by either B.1.1.7 or B.1.351 among fully vaccinated individuals. “Even though there were breakthrough infections, they didn’t lead to hospitalization and death, except very, very rarely,” says Abu-Raddad. Two people died of COVID-19 caused by B.1.351 after receiving their second vaccine dose, but it is very likely that they were infected before the protective effects of the booster shot began. “If, a year ago, I told somebody we would have 75% effectiveness against the worst variants we had, they would consider this extremely good news,” Abu-Raddad adds. Promising data Shabir Madhi, a vaccinologist at the University of the Witwatersrand in Johannesburg, South Africa, says the Qatari results are promising. The comparatively high levels of virus-blocking antibodies triggered by two doses of an RNA vaccine probably explain why it confers better protection against B.1.351 than do other vaccines, such as the one developed by the University of Oxford, UK, and pharmaceutical company AstraZeneca in Cambridge, UK. But Madhi expects that other vaccines will also prevent severe disease caused by that variant. In another 5 May New England Journal of Medicine study2, his team reported that the jab produced by biotechnology company Novavax in Gaithersburg, Maryland, lowered the risk of getting COVID-19 by 60% in participants without HIV in a South African trial involving more than 6,000 people. As-yet unpublished data show that the vaccine was highly effective against severe cases of COVID-19 caused by B.1.351, with no cases in vaccinated individuals and five in the placebo arm. If vaccine efficacy is lower against B.1.351, even highly successful immunization programmes in countries affected by the variant might not reduce cases to the same extent as in countries dealing with less troublesome strains, says Madhi. “Nevertheless, by protecting high-risk individuals, we could still return to a relatively normal lifestyle, even with ongoing circulation.” Qatar, where more than one-third of the population has received at least one dose of the vaccine, might provide an early glimpse at how the worst coronavirus variants can be controlled. Abu-Raddad says there is evidence that the Pfizer–BioNTech vaccine might also be highly effective at blocking transmission of B.1.351. And after cases of the variant peaked in mid-April, he says, “things have been going extremely well, the numbers are going down very, very rapidly”. Publication cited available in NEJM (May 5, 2021): https://www.nejm.org/doi/10.1056/NEJMc2104974 See also The Lancet (May 5, 2021): https://doi.org/10.1016/S0140-6736(21)00947-8

|
Scooped by
Juan Lama
|
Moderna Inc (MRNA.O) said on Wednesday early human trial data shows that a third dose of either its current COVID-19 shot or an experimental new vaccine candidate increases immunity against variants of COVID-19 first found in Brazil and South Africa. The booster shots, given to volunteers previously inoculated with Moderna's two-dose vaccine regimen, also boosted antibodies against the original version of COVID-19, Moderna said. The early data comes from a 40-person trial testing both Moderna's existing shot and a version developed to protect against the South African variant of COVID-19 called mRNA-1273.351. Moderna is also studying a shot that combines both the new and existing vaccine. The results show that while booster shots of either version of the vaccine increased antibodies against all of the variants of COVID-19 tested in the trial, the new booster had a bigger response against the South African variant than the original vaccine. "We are encouraged by these new data, which reinforce our confidence that our booster strategy should be protective" against the newer variants of COVID-19, Stephane Bancel, Moderna's chief executive officer, said in a statement. Both booster shots were well tolerated, with side effects similar to what volunteers in previous studies experienced from the second dose of its vaccine, Moderna said. The new variants of COVID-19 first discovered in South Africa and Brazil are thought to be more resistant to existing vaccines. Both variants have been detected in the United States but comprise only a small fraction of U.S. cases so far, according to federal data last updated in April. Moderna’s study is looking at levels of antibodies in participants’ blood that combat COVID-19, an early indication that they will be protected against the virus. It first announced it was studying ways to protect against the variants of COVID-19 in February. Moderna expects to share additional data soon on another potential booster shot that mixes its existing COVID-19 vaccine with the newly developed shot. The U.S. government scientists at the National Institute of Allergy and Infectious Diseases (NIAID) is conducting a separate early stage study of mRNA-1273.351, Moderna said. Moderna's Press Release (May 5, 2021): https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-initial-booster-data-against-sars-cov

|
Scooped by
Juan Lama
|
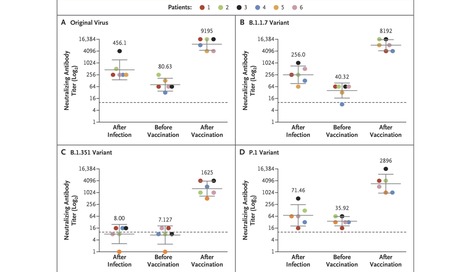
The BNT162b2 vaccine was shown to have 95% efficacy against coronavirus disease 2019 (Covid-19).1 To date, the two-dose vaccine protocol has not been approved in Israel for persons previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); however, administration of a single dose is now being considered. In addition to the original virus first identified in Wuhan, China, SARS-CoV-2 variants first identified in the United Kingdom (B.1.1.7), South Africa (B.1.351), and Brazil (P.1) have been detected in recent months.2 Samples from persons who had been vaccinated or previously infected with the original virus or the B.1.1.7 variant were shown to have significantly less neutralizing activity against the B.1.351 variant than against the other variants.3,4 In this study, we investigated whether one dose of the BNT162b2 vaccine would increase neutralizing activity against the B.1.1.7, B.1.351, and P.1 variants in persons previously infected with SARS-CoV-2. A microneutralization assay with isolates of the original virus (sublineage B.1) and the B.1.1.7, B.1.351, and P.1 variants was performed on 18 serum samples from six health care workers previously infected with SARS-CoV-2, with a sample obtained from each patient at three time points: 1 to 12 weeks after natural infection, immediately before vaccination, and 1 to 2 weeks after vaccination (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). All six health care workers were women (32 to 67 years of age) and had been infected with the original virus (sublineage B.1), as determined by sequencing of SARS-CoV-2 performed at the time of diagnosis. Samples obtained at the first time point had neutralizing activity against the original virus and the B.1.1.7 and P.1 variants, with geometric mean titers of 456, 256, and 71, respectively, but had little or no neutralizing activity against the B.1.351 variant, with a geometric mean titer of 8. At the second time point, geometric mean titers were 81, 40, 36, and 7 for the original virus and the B.1.1.7, P.1, and B.1.351 variants, respectively. Of note, at the third time point, geometric mean titers were 9195, 8192, 2896, and 1625 for the original virus and the B.1.1.7, P.1, and B.1.351 variants, respectively — that is, the titers after vaccination were 114, 203, 81, and 228 times as high as the titers immediately before vaccination (Figure 1 and Table S2). This study showed that, in our small cohort, one vaccine dose substantially increased neutralizing activity against all variants tested, with similar titers detected across patients for each variant. This highlights the importance of vaccination even in previously infected patients, given the added benefit of an increased antibody response to the variants tested. Limitations of the study include the small cohort of only women and the lack of evaluation of T-cell response. However, we think the fact that all six patients responded similarly to vaccination supports our conclusions. Further studies could investigate the effects of a second vaccine dose on neutralizing activity against variants of concern in persons who have and persons who have not been previously infected. Published in New England J. Medicine (April 7, 2021): https://10.1056/NEJMc2104036

|
Scooped by
Juan Lama
|
Infected rodents pose no immediate danger to humans, but the research suggests that mutations are helping the coronavirus expand its range of potential hosts. Bats, humans, monkeys, minks, big cats and big apes — the coronavirus can make a home in many different animals. But now the list of potential hosts has expanded to include mice, according to an unnerving new study. Infected rodents pose no immediate risk to people, even in cities like London and New York, where they are ubiquitous and unwelcome occupants of subway stations, basements and backyards. Still, the finding is worrying. Along with previous work, it suggests that new mutations are giving the virus the ability to replicate in a wider array of animal species, experts said. “The virus is changing, and unfortunately it’s changing pretty fast,” said Timothy Sheahan, a virologist at the University of North Carolina at Chapel Hill, who was not involved in the new study. In the study, the researchers introduced the virus into the nasal passages of laboratory mice. The form of the virus first identified in Wuhan, China, cannot infect laboratory mice, nor can B.1.1.7, a variant that has been spreading across much of Europe, the researchers found. But B.1.351 and P1, the variants discovered in South Africa and Brazil, can replicate in rodents, said Dr. Xavier Montagutelli, a veterinarian and mouse geneticist at the Pasteur Institute in Paris, who led the study. The research, posted online earlier this month, has not yet been reviewed for publication in a scientific journal. The results indicate only that infection in mice is possible, Dr. Montagutelli said. Mice caught in the wild have not been found to be infected with the coronavirus, and so far, the virus does not seem to be able to jump from humans to mice, from mice to humans, or from mice to mice. “What our results emphasize is that it is necessary to regularly assess the range of species that the virus can infect, especially with the emergence of new variants,” Dr. Montagutelli said. The coronavirus is thought to have emerged from bats, with perhaps another animal acting as an intermediate host, and scientists worry that the virus may return to what they describe as an animal “reservoir.” Apart from potentially devastating those animal populations, a coronavirus spreading in another species may then acquire dangerous mutations, returning to humans in a form the current vaccines weren’t designed to fend off. Minks are the only animals known to be able to catch the coronavirus from humans and pass it back. In early November, Denmark culled 17 million farmed mink to prevent the virus from evolving into dangerous new variants in the animals. More recently, researchers found that B.1.1.7 infections in domesticated cats and dogs can cause the pets to develop heart problems similar to those seen in people with Covid-19. To establish a successful infection, the coronavirus must bind to a protein on the surface of animal cells, gain entry into the cells, and exploit their machinery to make copies of itself. The virus must also evade the immune system’s early attempts at thwarting the infection. Given all those requirements, it is “quite extraordinary” that the coronavirus can infect so many species, said Vincent Munster, a virologist at the National Institute of Allergy and Infectious Diseases. “Typically, viruses have a more curtailed host range.” Mice are a known reservoir for hantavirus, which causes a rare and deadly disease in people. Even though the coronavirus variants don’t seem to be able to jump from mice to people, there is potential for them to spread among rodents, evolve into new variants, and then infect people again, Dr. Munster said. The variants may also threaten endangered species like black-footed ferrets. “This virus seems to be able to surprise us more than anything else, or any other previous virus,” Dr. Munster said. “We have to err on the side of caution.” Dr. Sheahan said he was more concerned about transmission to people from farm animals and pets than from mice. “You’re not catching wild mice in your house and snuggling — getting all up in their face and sharing the same airspace, like maybe with your cat or your dog,” he said. “I’d be more worried about wild or domestic animals with which we have a more intimate relationship.” But he and other experts said the results emphasized the need to closely monitor the rapid changes in the virus. “It’s like a moving target — it’s crazy,” he added. “There’s nothing we can do about it, other than try and get people vaccinated really fast.” Preprint available in bioRxiv (March 18, 2021):

|
Scooped by
Juan Lama
|
The emergence of SARS-CoV-2 variants with enhanced transmissibility, pathogenesis and resistance to vaccines presents urgent challenges for curbing the COVID-19 pandemic. While Spike mutations that enhance virus infectivity may drive the emergence of these novel variants, studies documenting a critical a role for interferon responses in the early control of SARS-CoV-2 infection, combined with the presence of viral genes that limit these responses, suggest that interferons may also influence SARS-CoV-2 evolution. Here, we compared the potency of 17 different human interferons against 5 viral lineages sampled during the course of the global outbreak that included ancestral and emerging variants. Our data revealed increased interferon resistance in emerging SARS-CoV-2 variants, indicating that evasion of innate immunity is a significant driving force for SARS-CoV-2 evolution. These findings have implications for the increased lethality of emerging variants and highlight the interferon subtypes that may be most successful in the treatment of early infections. Posted in bioRxiv (March 21, 2021): https://doi.org/10.1101/2021.03.20.436257

|
Scooped by
Juan Lama
|
- Pfizer's and Moderna's shots were at least 10 times less effective against variant in a new study.
- Researchers tested the vaccines on a variant first found in South Africa, which is now in 20 states.
- A mutation on the variant called E484K appeared to be a "major contributor," the study authors said.
COVID-19 vaccines from Moderna and Pfizer-BioNTech appear significantly less effective against the coronavirus variant first found in South Africa, a lab study has suggested. The percentage of protective antibodies that neutralized the variant — called B.1.351, which has been recorded in 20 US states — was 12.4 fold lower for Moderna's COVID-19 shot than against the original coronavirus, and 10.3 fold lower for Pfizer's, the study authors said. This was a bigger drop than in previous lab studies testing the vaccines against manufactured forms of the variant, they said. For this study, the researchers used real forms of the variant taken from people who had caught the virus. Both the Pfizer and Moderna vaccines have been authorized for emergency use in the US. B.1.351 was first detected in South Africa in October 2020. It has since spread to 42 countries, including to the US, where it is circulating in at least 20 states, including California and Texas, the Centers for Disease Control and Prevention said. There are 81 reported cases of B.1.351 in the US overall, the CDC said. The researchers found that the antibody activity from both vaccines was "essentially unchanged" against the variant first found in the UK, B.1.1.7. There are 3,037 reported cases in the US of B.1.1.7, the CDC said, and experts believe it will soon become the dominant strain in the US. The scientists, from Columbia University, also tested lab-made viruses that had certain mutations. They said that one specific mutation, E484K, appeared to be a "major contributor" to the B.1.351 variant's ability to evade the antibody response. E484K is not usually present in B.1.1.7, the variant first found in the UK. The study has been accepted by science journal Nature but not yet published. Taking samples from the real world In the experiment, scientists took 10 blood samples from people who had received two doses of Pfizer's vaccine, 28 days after their second dose, and 12 samples from those who had received two doses of Moderna's vaccine, 43 days after their second dose. They then compared how well antibodies in the blood samples "neutralized" the original coronavirus, compared to real-life B.1.1.7 and B.1.351 coronavirus variants. The sample size was small, and the antibody response is just one aspect of the immune response, so it remains unclear how well the vaccines work against the variant first found in South Africa in real life. Pfizer has conducted petri-dish tests before that showed a less potent antibody response against a lab-made coronavirus variant that mimicked the variant first found in South Africa. It was not the exact B.1.351 variant. The study, published as correspondence to the New England Journal of Medicine February 17 and updated March 8, suggested the vaccine would work against the variant. It also showed that the antibody response from Pfizer's shot held up against the variant first found in Brazil, P.1, that has a similar set of mutations to B.1.351. Moderna ran similar tests and said that its vaccine held up well against the mutations found in B.1.1.7, the variant first found in the UK, but less well against the mutations found in B.1.351, the variant first identified in South Africa. Again, it used lab-made variants. Both companies said in January that they were developing booster shots specifically to tackle the B.1.351 variant. Neither of the vaccines has been properly tested against the variant first found in South Africa in the real world. In Israel, Pfizer's vaccine has been shown to be highly effective against the B.1.1.7 variant, first found in the UK. About 80% of Israelis with COVID-19 are infected with B.1.1.7. The COVID-19 vaccine from Johnson & Johnson was less effective in the clinical trials that took place in South Africa. Original Findings to Be Published in Nature (March 8, 2021):

|
Scooped by
Juan Lama
|
Doctors in France are treating a critically ill patient infected with the South African coronavirus variant, four months after he recovered from COVID-19, in what study authors said was the first case of its kind. The 58-year-old man had a history of asthma and initially tested positive for COVID-19 in September when he presented to medical staff with a fever and shortness of breath. The symptoms persisted only for a few days, and the man tested negative for COVID-19 twice in December 2020. However, he was admitted to hospital in January and diagnosed with the South African variant. The patient's condition worsened, and he is currently in a "critical condition" on a ventilator. "This is, to our knowledge, the first description of reinfection with the South African (variant) causing severe COVID-19, four months after a first mild infection," said authors of a study published this week in the journal Clinical Infectious Diseases. The 501Y.V2 coronavirus variant emerged late last year in South Africa and immediately provoked alarm among disease specialists. It has eight key mutations, one of which affects the virus' spike protein, making it more effective at binding to human cells and therefore more infectious. Vaccine manufacturers Pfizer/BioNTech and Moderna say their mRNA vaccines retain their effectiveness against the South African variants and another that emerged last year in Britain. However a study last week showed that AstraZeneca's vaccine failed to prevent mild and moderate cases of infection of the South African variant. "The impact of 501Y.V2 mutations on the effectiveness of vaccines developed based on earlier SARS-CoV-2 strains is still unknown," said the authors of the reinfection study. Case study published in Clinical Infectious Diseases (Feb. 10, 2021): https://doi.org/10.1093/cid/ciab129

|
Scooped by
Juan Lama
|
The bad news, coming nearly a week after a million doses of the AstraZeneca-Oxford vaccine arrived in South Africa, was a big setback for the country. South Africa halted use of the AstraZeneca-Oxford coronavirus vaccine on Sunday after evidence emerged that the vaccine did not protect clinical trial volunteers from mild or moderate illness caused by the more contagious virus variant that was first seen there. The findings were a devastating blow to the country’s efforts to combat the pandemic. Scientists in South Africa said on Sunday that a similar problem held for people who had been infected by earlier versions of the coronavirus: The immunity they acquired naturally did not appear to protect them from mild or moderate cases when they were reinfected by the variant, known as B.1.351. The developments, coming nearly a week after a million doses of the AstraZeneca-Oxford vaccine arrived in South Africa, were an enormous setback for the country, where more than 46,000 people are known to have died from the virus They were also another sign of the dangers posed by new mutations in the coronavirus. The B.1.351 variant has spread to at least 32 countries, including the United States. The number of cases evaluated as part of the studies outlined by South African scientists on Sunday were low, making it difficult to pinpoint just how effective or not the vaccine might be against the variant. And because the clinical trial participants who were evaluated were relatively young and unlikely to become severely ill, it was impossible for the scientists to determine if the variant interfered with the AstraZeneca-Oxford vaccine’s ability to protect against severe Covid-19, hospitalizations or deaths. The scientists said, however, that they believed the vaccine might protect against more severe cases, based on the immune responses detected in blood samples from people who were given it. If further studies show that to be the case, South African health officials will consider resuming use of the AstraZeneca-Oxford vaccine, they said. The new research findings have not been published in a scientific journal. But the discovery that the AstraZeneca-Oxford product showed minimal efficacy in preventing mild and moderate cases of the new variant added to the mounting evidence that B.1.351 makes current vaccines less effective. Pfizer and Moderna have both said that preliminary laboratory studies indicate that their vaccines, while still protective, are less effective against B.1.351. Novavax and Johnson & Johnson have also sequenced test samples from their clinical trial participants in South Africa, where B.1.351 caused the vast majority of cases, and both reported lower efficacy there than in the United States. “These results are very much a reality check,” Shabir Madhi, a virologist at University of the Witwatersrand who ran the AstraZeneca-Oxford vaccine trial in South Africa, said of the findings released on Sunday. The pause in the country’s rollout of the AstraZeneca-Oxford vaccine means that the first shipments will now be put in warehouses. Instead, South African health officials said they would inoculate health workers in the coming weeks with the Johnson & Johnson vaccine, which has shown strong efficacy in preventing severe cases and hospitalizations caused by the new variant. Johnson & Johnson has applied for an emergency use authorization in South Africa. But health officials there indicated that even before it is authorized, some health workers could be given the vaccine as part of an ongoing trial. In the AstraZeneca-Oxford trial in South Africa, roughly 2,000 participants were given either two doses of the vaccine or placebo shots. There was virtually no difference in the numbers of people in the vaccine and placebo groups who were infected with B.1.351, suggesting that the vaccine did little to protect against the new variant. Nineteen of the 748 people in the group that was given the vaccine were infected with the new variant, compared with 20 out of 714 people in the group that was given a placebo. That equates to a vaccine efficacy of 10 percent, though the scientists did not have enough statistical confidence to know for sure whether that figure would hold among more people. Researchers also conducted laboratory experiments on blood samples from people who had been vaccinated and found a significant reduction in the activity levels of vaccine-generated antibodies against the B.1.351 variant compared with other lineages. Beyond the troubling news about the AstraZeneca-Oxford vaccine, Dr. Madhi reported evidence suggesting that past infection by earlier versions of the coronavirus did not protect people in South Africa from the B.1.351 variant. In order to determine who had previously been infected by the coronavirus, researchers tested blood samples from people who had enrolled in a trial of the Novavax vaccine, but who were given placebo shots and not the vaccine itself. The researchers compared the levels of infection by the new variant in people who showed evidence of having previously had Covid-19 with the levels of infection in people who did not, and found no difference. That suggested, Dr. Madhi wrote on a slide presented Sunday night, that “past infection by ‘original’ variants of SARS-CoV-2 do NOT protect against mild and moderate Covid-19 from the B.1.351 variant.....” University of Witwatersrand, Johannesburg (Feb. 7, 2021): https://www.wits.ac.za/covid19/covid19-news/latest/oxford-covid-19-vaccine-trial-results.html

|
Scooped by
Juan Lama
|
An early analysis in Britain found that the vaccine had an efficacy rate of nearly 90 percent. But in a small South Africa trial, the efficacy rate dropped to just under 50 percent. Novavax, a little-known company supported by the U.S. federal government’s Operation Warp Speed, said for the first time on Thursday that its Covid-19 vaccine offered robust protection against the virus. But it also found that the vaccine is not as effective against the fast-spreading variant first discovered in South Africa, another setback in the global race to end a pandemic that has already killed more than 2.1 million people. That could be a problem for the United States, which hours earlier reported its first known cases of the contagious variant in two unrelated people in South Carolina. And it came just days after Moderna and Pfizer said that their vaccines were also less effective against the same variant. Novavax, which makes one of six vaccine candidates supported by Operation Warp Speed last summer, has been running trials in Britain, South Africa, the United States and Mexico. It said Thursday that an early analysis of its 15,000-person trial in Britain revealed that the two-dose vaccine had an efficacy rate of nearly 90 percent there. But in a small trial in South Africa, the efficacy rate dropped to just under 50 percent. Almost all the cases that scientists have analyzed there so far were caused by the variant, known as B.1.351. The data also showed that many trial participants were infected with the variant even after they had already had Covid. “We have the first trial — we are the first to conduct an efficacy trial — in the face of a changing virus,” said Stanley Erck, the president and chief executive of Novavax. He said that researchers expected the variants could change the trial results, but “the amount of change has been a bit of a surprise to everyone.” The South Africa trial was relatively small — with just 4,400 volunteers — and was not designed to come up with a precise estimate of how much protection the vaccine provides. Still, the results were striking enough that the company said it would soon begin testing a new vaccine tailored to protect against the variant from South Africa. “You’re going to have to make new vaccines,” Mr. Erck said. John Moore, a virologist at Weill Cornell Medicine who was not involved in the studies, praised the results. “Fifty percent is not as good as 100, but it’s a damn sight better than zero,” he said, noting that given the strong results in Britain, it was likely very similar in efficacy to the Pfizer and Moderna vaccines. While the Pfizer and Moderna vaccines rely on a newer mRNA technology that has not been used in previous vaccines, Novavax’s candidate employs an older, more established method that relies on injecting coronavirus proteins to provoke an immune response. The fact that three vaccines all appeared to show lowered effectiveness against the variant from South Africa is not encouraging, and the results Novavax announced Thursday were the first to occur outside of a laboratory, testing how well a vaccine worked in people infected with a new variant. Johnson & Johnson is also on the cusp of announcing results of its Covid-19 vaccine trials, and has also tested its candidate in South Africa. The announcement from Novavax raises the stakes for Johnson & Johnson. The company was expected to announce its results as early as last weekend, and the delay has triggered speculation among scientists that the firm has also discovered that its vaccine worked less well in South African trial volunteers who were infected with the variant. In an earnings call on Tuesday, Alex Gorsky, the chief executive officer of the company, said they were looking forward to sharing results from their late-stage trial by early next week. The emergence of several highly contagious variants has complicated efforts to bring the pandemic under control, leading world leaders to shut down travel to places like Britain and South Africa even as the variants already appear to have circled the globe. In the United States, researchers have warned that the variant first identified in Britain, which is believed to be more infectious, could become the dominant form of the virus in this country by March. The United States is well behind other countries in testing for such variants, and the one from South Africa has been found in about 30 countries. But experts have also said there are reasons for optimism, noting that the vaccines remain effective. The best way to combat contagious new variants is to continue vaccination and other public health measures, which will slow the virus’s ability to infect new people and mutate further. “This is really worrisome,” said Dr. Peter Hotez, a vaccine expert at the Baylor College of Medicine and the inventor of a coronavirus vaccine. “We have to have the American people vaccinated by late in the spring or early summer to have any hope in preventing the South African and the U.K. variants from taking over.” Drug makers could update their vaccines and offer new shots at regular intervals, similar to the flu vaccine....... Novavax Press Release (Jan. 28, 2021): https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3
|



 Your new post is loading...
Your new post is loading...