Your new post is loading...

|
Scooped by
Juan Lama
|
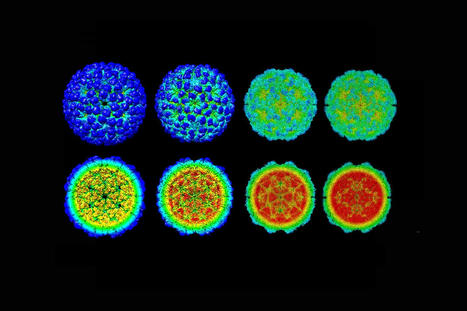
Understanding every step in the life cycle of a virus is crucial for identifying potential targets for treatment. Now, scientists at the Institute of Science and Technology (IST) Austria were able to show how a virus from the retrovirus family—the same family as HIV—protects its genetic information and becomes infectious. Furthermore, they show an unexpected flexibility of the virus. This study is published in the journal Nature Communications. Viruses are perfect molecular machines. Their only goal is to insert their genetic material into healthy cells and thus multiply. With deadly precision, they thereby can cause diseases that cost millions of lives and keep the world on edge. One example for such a virus, although currently less discussed, is HIV that causes the ongoing global AIDS-epidemic. Despite the progress made in recent years, 690 000 people died in 2019 alone as a result of the virus infection. "If you want to know the enemy, you have to know all its friends," says Martin Obr, postdoc at the Schur group at IST Austria. Together with his colleagues, he therefore studies a virus belonging to the same family as HIV—the Rous sarcoma virus, a virus causing cancer in poultry. With its help, he now gained new insights into the important role a small molecule plays in the assembly of these type of viruses. Protecting the virus blueprint In their study, published in the journal Nature Communications, the team together with collaborators at Cornell University and the University of Missouri focused on the late phase of retrovirus replication. "It is a long way from an infected cell to the mature virus particle that can infect another cell," explains first author Martin Obr. A new particle buds from the cell in an immature, non-infectious state. It then forms a protective shell, a so-called capsid, around its genetic information and becomes infectious. This protective shell consists of a protein, which is organized in hexamers and a few pentamers. The team discovered that a small molecule called IP6 plays a major role in stabilizing the protein shell within the Rous sarcoma virus. "If the protective shell is not stable, the genetic information of the virus could be released prematurely and will be destroyed, but if it's too stable the genome can't exit at all and, therefore, becomes useless," says Assistant Professor Florian Schur. In a previous study, he and his colleagues were able to show IP6 is important in the assembly of HIV. Now, the team proved it to be as important in other retroviruses showing just how essential the small molecule is in the virus life cycle. "When building a car, you have all these big metal parts, like the hood, the roof and the doors—the screws are connecting everything. In our case, the big parts are the capsid proteins and the IP6 molecules are the screws," says Obr. Unexpected flexibility Further developing cryo-electron tomography, a technique that allows scientists to look at extremely small samples in their natural state, the team was able to see how variable the shapes formed by capsid proteins are. "Now we ask ourselves: Why does the virus change the shape of its capsid? What is it adapting to?" says postdoc Martin Obr. Different capsid shapes within the same type of virus could point to differences in the infectivity of virus particles. "Whatever happens, happens for a reason, but there is no clear answer yet," says Florian Schur. Further developing the technology to get to the bottom of these highly optimized pathogens remains a challenging and fascinating task for the scientists. Published in Nature Commun. (May 28, 2021) https://doi.org/10.1038/s41467-021-23506-0

|
Scooped by
Juan Lama
|
Neuroscientists at Lund University in Sweden have developed a new technology that engineers the shell of a virus to deliver gene therapy to the exact cell type in the body that needs to be treated. The researchers believe that the new technology can be likened to dramatically accelerating evolution from millions of years to weeks. Several of the new revolutionary treatments that have been used clinically in recent years to treat complex diseases—such as spinal muscular atrophy and enzyme deficiency—are based on gene therapy. With gene therapy, the genetic material is controlled or altered using biological drugs. Examples of this are the gene scissors CRISPR / Cas9 and the so-called CAR-T cells that are used to treat various forms of cancer. This type of treatment is often engineered by growing viruses in the laboratory. The viruses are altered so that they are harmless and can deliver new genetic material to the body's cells, replacing the damaged genome. The virus's own genome, which is required for it to spread, has been completely removed. In the last five years, neuroscientist Tomas Björklund and his research group have developed a process that tailors these virus shells, or virus capsids, so that they can reach precisely the cell type in the body that needs to be treated, for example nerve cells. The process combines powerful computer simulations and modeling with the latest gene technology and sequencing technology. "Thanks to this technology, we can study millions of new virus variants in cell culture and animal models simultaneously. From this, we can subsequently create a computer simulation that constructs the most suitable virus shell for the chosen application– in this case, the dopamine-producing nerve cells for the treatment of Parkinson's disease," says Tomas Björklund, senior lecturer in translational neuroscience at Lund University. "You can view this as dramatically speeding up evolution from millions of years to weeks. The reason we can do this is that we study each "generation" of the virus in parallel with all the others in the same nerve cells. Unlike evolution, where only the best suited live on to the next generation, we can also learn what makes the virus work less well through this process. This is crucial when building computer models that interpret all the information," he continues. With the new method, researchers have been able to significantly reduce the need for laboratory animals, as millions of variants of the same drug are studied in the same individual. They have also been able to move important parts of the study from animals to cell culture of human stem cells. "We believe that the new synthetic virus we succeeded in creating would be very well suited for gene therapy for Parkinson's disease, for example, and we have high hopes that these virus vectors will be able to be put into clinical use. Together with researchers at Harvard University, we have established a new biotechnology company in Boston, Dyno Therapeutics, to further develop the virus engineering technology, using artificial intelligence, for future treatments," concludes Tomas Björklund. Published in P.N.A.S. (December 9, 2019): https://doi.org/10.1073/pnas.1910061116

|
Scooped by
Juan Lama
|
For the first time, Harvard researchers have captured images of individual viruses forming, offering a real-time view into the kinetics of viral assembly. The research is published in the Proceedings of the National Academy of Sciences. “Structural biology has been able to resolve the structure of viruses with amazing resolution, down to every atom in every protein,” said Vinothan Manoharan, the Wagner Family Professor of Chemical Engineering and Professor of Physics at the Harvard John A. Paulson School of Engineering and Applied Sciences. “But we still didn’t know how that structure assembles itself. Our technique gives the first window into how viruses assemble and reveals the kinetics and pathways in quantitative detail.” Manoharan is also co-director of the Quantitative Biology Initiative, a cross-Harvard effort that brings together biology, novel measurement techniques, statistics, and mathematics to develop causal, predictive mathematical models of biological systems. Manoharan and his team focused on single-stranded RNA viruses, the most abundant type of virus on the planet. In humans, RNA viruses are responsible for, among others, West Nile fever, gastroenteritis, polio, the common cold, and hand, foot, and mouth disease. These viruses tend to be very simple. The virus Manoharan and his team studied, which infects E. coli bacteria, is about 30 nanometers in diameter and has one piece of RNA, with about 3,600 nucleotides and 180 identical proteins. The proteins arrange themselves into hexagons and pentagons to form a soccer-ball-like structure around the RNA, called a capsid. How those proteins manage to form that structure is the central question in virus assembly. Until now, no one had been able to observe viral assembly in real time because viruses and their components are very small and their interactions are very weak. To observe the viruses, the researchers used an optical technique known as interferometric scattering microscopy, in which the light scattered off an object creates a dark spot in a larger field of light. The technique doesn’t reveal the virus’ structure but it does reveal its size and how that changes with time. The researchers attached viral RNA strands to a substrate, like stems of a flower, and flowed proteins over the surface. Then, using the interferometric microscope, they watched as dark spots appeared and grew steadily darker until they were the size of full-grown viruses. By recording intensities of those growing spots, the researchers could actually determine how many proteins were attaching to each RNA strand over time. “One thing we noticed immediately is that the intensity of all the spots started low and then shot up to the intensity of a full virus,” Manoharan said. “That shooting up happened at different times. Some capsids assembled in under a minute, some took two or three, and some took more than five. But once they started assembling, they didn’t backtrack. They grew and grew and then they were done.” Published in P.N.A.S on September 30, 2019: https://doi.org/10.1073/pnas.1909223116

|
Scooped by
Juan Lama
|
New findings reveal many different structural models, which can eventually lead to developing more targeted antiviral vaccines. New research reveals that the way viruses were perceived in terms of their architecture will need to be retooled, because they are actually structured in many more patterns than previously understood. The findings could have significant impact on how they are classified, our understanding of how they form, evolve and infect hosts, and strategies to identify ways to design vaccines to target them. In the 1950s and ’60s as scientists began to obtain high resolution images of viruses, they discovered the detailed structure of the capsid – an outer protective layer composed of multiple copies of the same protein – which protects the virus’ genetic material. The majority of viruses have capsids that are typically quasi-spherical and display icosahedral symmetry – like a 20-sided dice for instance. The capsid shell is what protects them, and as scientists discovered their structure, they proposed that capsids could have different sizes and hold different amounts of genome, and therefore could infect hosts differently. Why this matters When designing drugs to target viruses, scientists can now take their varying structural shapes into account to improve efficacy. Two researchers who study the structures of viruses, Antoni Luque, a theoretical biophysicist at San Diego State University and a member of its Viral Information Institute, and Reidun Twarock, a mathematical biologist from the University of York, UK, and a member of York’s Cross-disciplinary Centre for Systems Analysis, show that many viruses have essentially been misclassified for 60 years, including common viruses such as Herpes simplex and Zika. This was because despite having the structural images from cryo-electron microscopy, we did not have the mathematical description of many of the architectures of different viruses. “We discovered six new ways in which proteins can organize to form icosahedral capsid shells,” Luque said. “So, many viruses don’t adopt only the two broadly understood capsid architectures. There are now at least eight ways in which their icosahedral capsids could be designed.” Biotech applications Structural biologists can now take this information and reclassify the structure of the viruses, which will help unveil molecular and evolutionary relationships between different viruses. It will also provide a guide to engineer new molecular containers for nanotech and biotech applications, and it will help scientists to identify specific strategies to target the assembly of proteins in the capsid. This can eventually lead to a more systematic approach to developing antiviral vaccines. “We can use this discovery to target both the assembly and stability of the capsid, to either prevent the formation of the virus when it infects the host cell, or break it apart after it’s formed,” Luque said. “This could facilitate the characterization and identification of antiviral targets for viruses sharing the same icosahedral layout.” This new framework accommodates viruses that were previously outliers, provides new predictions of viral capsid architectures, and has identified common geometrical patterns among distant evolutionary related viruses that infect everyone from humans to bacteria. Twarock said the new blueprints also provide “a new perspective on viral evolution, suggesting novel routes in which larger and more complex viruses may have evolved from simple ones at evolutionary timescales.” Published on Sept. 27, 2019 in Nature Communications: https://doi.org/10.1038/s41467-019-12367-3
|

|
Scooped by
Juan Lama
|
A critical process in the infection cycle of viruses has been revealed for the first time in dynamic detail using pioneering plant-based technology. Evidence about the process of virus maturation revealed in the research could help us develop new methods for treating viral infections. Maturation plays a critical role for all animal and bacterial viruses and is required to produce infectious virions or particles. Though the outlines of the process have been determined for many groups of viruses, detailed mechanistic studies have not been reported. To provide the first detailed mechanistic study of maturation, Roger Castells-Graells, a rotation Ph.D. student working in Professor. George Lomonossoff’s laboratory at the John Innes Centre infiltrated genetic material of the insect virus Nudaurelia capensis omega virus (N?V) into dwarf tobacco plants N.benthamiana. This transient expression technique uses Virus Like Particles (VLPs) which are mimics of the authentic virus. The capsid or protein coat of the virus is produced by plant cells and the research team then analyses the material purified from infiltrated leaves. The research demonstrated that maturation of procapsids – immature viral structures – can occur within plant cells to yield fully functional mature capsids. This has not been observed previously in the absence of a natural infection and is a new application for the transient expression system pioneered by Professor Lomonossoff at the John Innes Centre. Comparative cryo-EM analysis of the structures of the procapsids and mature capsids revealed the large structural rearrangements both inside and between the protein subunits of the capsid that accompany maturation. These shape changes enable the chemical reactions that are necessary for the virus to infect the host. Professor Lomonossoff, a group leader at the John Innes Centre, said: “Most structural studies of virus particles to date have given a static picture of the particles. By isolating particles from plants that are undergoing the process of maturation, we have managed to obtain a picture of the dynamics of an essential part of a virus infection cycle.” The present study, a collaboration involving scientists at the University of Leeds, in Brazil and the USA, as well as at the John Innes Centre, reveals details of the structures at the beginning and the end of the maturation process. What is now required is an analysis of intermediate steps to get a complete understanding of the dynamics. This will enable the research team to determine the 3-D structures of intermediates in the maturation process to create a “movie.” “We have shown that maturation occurs over time within plant cells and that means we have discovered a valuable tool for studying virus maturation. We hope it will be of interest to potential collaborators and industry,” said Professor Lomonossoff. Plant-expressed virus-like particles reveal the intricate maturation process of a eukaryotic virus appears in Communications Biology Research Published in Communications Biology (May 24, 2021): http://dx.doi.org/10.1038/s42003-021-02134-w

|
Scooped by
Juan Lama
|
Gilead will now initiate two new clinical trials, for both treatment-naïve and multi-drug resistant HIV-1. Gilead has presented Phase I data from its trial of GS-6207, an investigational first-in-class inhibitor of HIV-1 capsid function. The data supports the drug’s further further development and potential role as a component in a long-acting HIV combination therapy. The data, taken from two Phase I studies, demonstrates that GS-6207 has potent antiviral activity and a potential dosing interval of up to every six months, and that the drug is generally well tolerated. Additionally, the company stated that in vitro virology study results suggest the investigational therapy could potentially be used in a broad range of people living with HIV regardless of their treatment history. The successful data “reinforce the potential of HIV capsid inhibition as a new long-acting therapeutic pathway to achieving durable viral suppression and support further clinical development of GS-6207,” said Diana Brainard, senior vice president, HIV and emerging viruses. “Based on these promising results, we look forward to initiating additional studies to evaluate GS-6207 in people living with HIV later this year.” On the news, the company will be initiating enrollment of two new clinical trials of GS-6207 in combination with other antiretroviral agents in people living with HIV. A Phase II and Phase II/III study, for patients living with treatment-naïve and multi-drug resistant HIV-1, respectively. Gilead Press Release available at: https://www.gilead.com/news-and-press/press-room/press-releases/2019/11/gilead-presents-data-on-investigational-hiv-1-capsid-inhibitor-gs-6207-as-a-potential-component-of-long-acting-hiv-therapy

|
Scooped by
Juan Lama
|
Like a janitor thumbing through a keychain to find just the right key to open a lock, the "rock-and-roll" motion of the canine parvovirus during the binding process may help explain how the virus can find the spot on a receptor to infect not just dogs, but multiple species, according to an international team of researchers. The model also could lead to a better understanding of how viruses enter a human body. The researchers, who report their findings today (Sept. 23) in the Proceedings of the National Academy of Sciences, used a sophisticated electron microscope that can take pictures of structures at the atomic level to examine the virus as it interacted with the transferrin receptor, or TfR, a protein on the surface of the cell that helps manage a body's iron uptake. "The virus uses the same receptor in many different species of animals," said Susan Hafenstein, associate professor of biochemistry and molecular biology and an affiliate member of the Huck Institutes of Life Sciences. "All of these animals—including raccoon, mink, cats, dogs—have a transferrin receptor, and, as you can imagine, the receptor would be slightly different depending on the species. So how is a virus able to use the receptor from a fox and the same receptor from a wolf or a cat?" The key may be motion that is generated by the virus and receptor molecules when they interact. The study shows that when the molecules interact, they can sway, which allows the virus to roll around the receptor point-of-contact searching for the right place to bind, according to Hafenstein, who is also an associate of the Institute for CyberScience, which provides Penn State faculty access to supercomputer resources. Once it binds, the virus hijacks the iron-uptake process that TfR regulates. "TfR's job is to bind iron-loaded transferrin," said Hafenstein. "So that when that iron-loaded transferrin binds to the receptor, it signals the receptor: 'OK. It's time to get into the cell.' At that point, the virus hitches a ride. For us, this was pretty exciting because it makes so much biological sense. If the virus binds naked TfR, it would just sit at the cell surface, but it prefers the TfR that's about to go in." Understanding the virus's versatility of attacking the receptor gives scientists a new understanding of how the disease swept through animal and pet populations in the 1970s. "Working with our collaborators at Cornell, we were able to see that the virus only needs two mutations on the surface to be able to jump from cats to dogs," said Hafenstein. "It was thought that it jumped from cats to dogs in the late seventies, causing a pandemic. However, with more work, our collaborators discovered that it's more complicated than that. Likely, it jumped from cats to raccoons to dogs." Hafenstein added that scientists were particularly baffled at the evolution of canine parvovirus because it is a DNA-based virus. "DNA viruses are not very well known for being able to jump species," said Hafenstein. "They don't exist with a lot of mutations, like RNA viruses such as HIV, which is treated with a drug cocktail because the virus mutates so rapidly."..... Published in P.N.A.S on Sept. 23, 2019: https://doi.org/10.1073/pnas.1904918116
|



 Your new post is loading...
Your new post is loading...