Your new post is loading...

|
Scooped by
Juan Lama
|
More than 12,000 people asked for tests in England after the BBC found 1,700 cases are undiagnosed. Up to 27,000 people caught it when they were given transfusions with infected blood from the 1970s until 1991. According to BBC analysis, a further 1,700 people who caught it in the same way have not yet been diagnosed. Left untreated, hepatitis can cause chronic liver disease and can be fatal. Known as the "silent killer", hepatitis C may cause few symptoms initially, with early signs including night sweats, brain fog, itchy skin and fatigue. But for every year a person carries the virus, their chance of dying from liver cirrhosis and related cancers increases. The Hepatitis C Trust told the BBC 12,800 people in England have requested NHS home-testing kits in just over a week, compared with 2,300 in the entire month of April. The charity said it had been "inundated with callers across the UK seeking further advice and testing". "It has been incredible seeing the response from the public as they have become more aware of the risks of hepatitis C," said Rachel Halford, from the charity. "Most people who get tested will receive a negative result and have peace of mind, but it is important to find those individuals who are unaware of their status so that we can get them access to a simple and effective treatment." The Sunday Times has reported Chancellor Jeremy Hunt will soon unveil a compensation package for those affected by the infected blood scandal. In an interview with the paper, Mr Hunt said he intended to fulfil a promise he made to a constituent who died after being affected by contaminated blood. An official announcement from the government is expected following the publication of the final report from the infected blood inquiry on Monday. Undiagnosed cases The BBC recently exclusively revealed the true scale of undiagnosed cases of the disease, related to the infected blood scandal. The BBC's calculation of 1,700 undiagnosed cases is based on statistics submitted to a public inquiry into the infected blood scandal, as well as Freedom of Information requests to infected blood support schemes. Official documents, seen by BBC News, revealed how the UK government and the NHS failed to adequately trace those who were most at risk of having the virus. The BBC also revealed how the authorities actively tried to limit the public's awareness of the virus to avoid embarrassing "bottlenecks" at hospital liver units. Testing was limited because of "resource implications for the NHS". Charlotte Dickens, 70, is among those to have asked for a home-testing kit following the publication of this story, and is awaiting the result. Ms Dickens, from Surrey, had a blood transfusion after suffering a haemorrhage during childbirth in 1980. She said she was "astounded" that she and others were not tested for the disease once the risks became clear. When news of the scandal first broke, she assumed she was not affected. "I had no idea it [hepatitis C]could linger around and cause liver cancer. Why didn't we all get tested, what's the answer to that? It is hard to find an excuse." Ms Dickens added that she felt she should speak up due to the many people who have died as a result of the scandal. About 3,000 people are known to have died as a result of receiving infected blood products. But it is believed that many people who unknowingly contracted hepatitis C have also died. Victoria Arkley recently told the BBC she was angry that her mother Maureen died from liver cancer soon after being diagnosed with hepatitis C. She believes she was infected during transfusions 47 years earlier: "Where was the public health campaign? Why didn't the doctor test her? They knew she had had transfusions, but no one tested her. I'm so angry." Most of those affected were people with blood disorders such as haemophilia, or people who had received blood transfusions. Due to a shortage of blood products in the UK, many were obtained from the US and had been bought from high-risk donors such as prisoners and people who misused drugs. Even though hepatitis C was not formally identified until 1989, health officials and NHS staff recognised that this form of hepatitis could be fatal as early as 1980.

|
Scooped by
Juan Lama
|
Elementary students lined up behind a white curtain in the middle of a grand gymnasium at their school in northern California. They stood still as a dog handler walked a yellow Labrador along the other side of the curtain. Hidden from the children’s view, the 2-year-old female pup sniffed each child’s shoes from beneath that curtain barrier. After each sniff, the dog looked back up at the handler. Then the handler brought the dog to the next tiny pair of feet beneath the curtain, and the dog curiously brought her snout close to those toes, then a young girl’s lavender tennis shoes and then another child’s white high-tops. The dog was smelling for what are called volatile organic compounds that are known to be associated with Covid-19 infections. While watching the Covid-sniffing dog in action, Dr. Carol Glaser saw her vision come to life. Months prior, Glaser and her team were implementing the school’s Covid-19 testing program, using antigen nasal swab tests. Around that same time, Glaser heard about reports of dogs being used to screen for Covid-19 infections in sports venues, airports and other public settings. That’s when Glaser had her “aha” moment – incorporating canines into Covid-19 testing programs at schools, nursing homes or other public facilities could help save time, personnel, possibly even costs, and “would be a lot more fun,” she said. “I thought if we had dogs in schools to screen the students it would be so much faster and less burdensome for schools,” said Glaser, assistant deputy director in Central Laboratory Services and medical officer for infectious disease laboratories at the California Department of Public Health. “Remember when an antigen test is done at school, as opposed to home, there’s a whole bunch of rules and regulations that run under that. It’s not as simple as just handing those things out at school and having the kids do them,” said Glaser, who oversaw antigen testing programs at some California public schools. For now, Glaser and her colleagues described in a new study the lessons they learned from the Covid-19 dog screening pilot program that they launched in some California K-12 public schools. In their research, published Monday in the journal JAMA Pediatrics, they wrote that the goal was to use dogs for screening and only use antigen tests on people whom the dogs screened as positive – ultimately reducing the volume of antigen tests performed by about 85%. They wrote that their study supports the “use of dogs for efficient and noninvasive” Covid-19 screening and “could be used for other pathogens.” Letting the dogs out for screening The dogs used in the pilot program – two yellow Labradors named Rizzo and Scarlett – trained for a couple of months in a laboratory, sniffing donated socks that were worn by people who either had Covid-19 or didn’t. The dogs alerted their handlers when they detected socks that had traces of the disease – and received a reward of either Cheerios or liver treats. “The one thing we do know for sure is when you’re collecting a sample off of a human being, you want to go where the most scent is produced. That is the head, the pits, the groin and the feet. Given those options, I went with feet,” said Carol Edwards, an author of the study and executive director of the nonprofit Early Alert Canines, which trains medical alert service dogs, including Rizzo and Scarlett. “We collected some socks from people willing to donate socks, and we taught the dogs, by smelling the socks, which ones were the Covid socks and they picked it up very quickly,” Edwards said. “Then we moved into the schools and started sniffing the kids at the ankles.” Last year, from April to May, the dogs visited 27 schools across California to screen for Covid-19 in the real world. They completed more than 3,500 screenings. Rizzo acted as an energized worker, performing tasks with eagerness, Edwards said, while Scarlett tended to have more of a mellow and easygoing personality. The screening process involves people – who voluntarily opted in to participate – standing 6 feet apart while the dogs, led by handlers, sniff each person’s ankles and feet. The dogs are trained to sit as a way of alerting their handlers that they detect a potential Covid-19 infection. To protect each person’s privacy, sometimes the people face away from the dogs and toward a wall or behind a curtain, so that they can’t see the dogs or when a dog sits. If the dog sits in between two people, the handler will verbally ask the dog, “Show me?” And the dog will move its snout to point toward the correct person. “Our dogs can come in, they can screen 100 kids in a half hour, and then only the ones the dog alerts on have to actually do a test,” Edwards said. “There’s no invasive nasal swab unless the dog happens to indicate on you.” The researchers found that the dogs accurately alerted their handlers to 85 infections and ruled out 3,411 infections, resulting in an overall accuracy of 90%. However, the dogs inaccurately alerted their handlers to infections in 383 instances and missed 18 infections, which means the dogs demonstrated 83% sensitivity and 90% specificity when it came to detecting Covid-19 infections in the study. “Once we stepped into the schools, we saw a drop in their specificity and sensitivity due to the change,” Edwards said, referring to the distractions that children in a school setting can bring. However, Edward said, accuracy improved as the dogs spent more times in schools. In comparison, Covid-19 BinaxNOW antigen tests have been shown in one real-world study to demonstrate 93.3% sensitivity and 99.9% specificity. That study was conducted in San Francisco and published in 2021 in The Journal of Infectious Diseases. “We never said the dogs will replace the antigen. This was a time for us to learn how they compared,” Glaser said. “We will always plan on doing some amount of backup testing, but the idea would be that the actual antigen testing would be a fraction of what it would currently be because of the dogs.” “To run these antigen testing programs at school, it’s taking a lot of school personnel resources, test cards as well as biohazard waste. So, I have no doubt in the long-run once it can be perfected, dogs will be cheaper, but I don’t have a great cost comparison,” she said. This isn’t the first time that dogs’ abilities to detect traces of Covid-19 infections in real-time have been studied in the scientific literature. “What we have learned in this work is that the dogs in general are capable of discriminating samples from individuals testing,” said Dr. Cindy Otto, professor and director of the Penn Vet Working Dog Center at the University of Pennsylvania, who was not involved in the new study. Regarding the new research, Otto said, “On the surface their results are encouraging and with the appropriate selection of dogs, rigorous training and impeccable quality control, there is the potential for dogs to be incorporated in threat monitoring.” Nursing homes could be next Now that Glaser and her colleagues have published research about their Covid-19 dog screening pilot program, she is eager to implement the approach in nursing home settings. “Honestly, schools aren’t that interested in testing anymore. The outbreaks just aren’t what they used to be, but what we have done is we’ve transitioned to nursing homes, because there is a tremendous need in nursing homes,” Glaser said, adding that many residents may prefer to undergo screening with a dog than with uncomfortable nasal swabs. “What would you rather have: A swab in your nose or something that just maybe tickles your ankle at most for testing?” In skilled nursing homes, the dogs visit each resident’s room to sniff their feet, calmly smelling for Covid-19 volatile organic compounds as the resident lies in bed or sits in a chair. “Thinking about where dogs would be deployed, I do really think nursing homes and residential care facilities and even schools – if they were ever to have a big outbreak – would be the natural next fit for this,” Glaser said. “We think we’ll probably end up primarily using them in nursing homes,” she said. “But we’re still doing a little bit of both – there was a school that asked us to come back last week.” The pilot program within California public schools also has left Edwards with hope for future opportunities in which canines can help detect disease in humans. “I really do think it’s the tip of the iceberg. This is the door swinging wide open, and now we need to collaborate with those in the science world and figure out where we can take this,” Edwards said. “There’s been a lot of chatter, even in the very beginning of this project, talking about what other diseases they could do. We’ve talked about TB, we’ve talked about flu A and B, possibly for this next flu season, seeing if we can get the dogs to alert on that,” she said, as volatile organic compounds are also produced by people with influenza. “It’s just a matter of being able to figure out how to collect samples, how to train the dogs, and then to be safe and effective around those diseases too.” Research published in JAMA Pediatrics (April 24, 2023): https://doi.org/10.1001/jamapediatrics.2023.0489

|
Scooped by
Juan Lama
|
Scientists are trying to develop tests that are just as convenient as antigen tests, but can tell people whether they currently have neutralizing antibodies that protect against SARS-CoV-2. As the world continues to learn how to live with Covid-19 in the long run, scientists are testing ways to quickly tell people how well-protected they are against the virus, and whether they need another booster. A new study, published Monday in Cell Reports Methods, presents a simple test to detect neutralizing antibodies against SARS-CoV-2, using little more than a finger prick and a testing cartridge. The approach, if it bears out in large-scale testing and receives the blessing of regulatory agencies, could one day offer a cheap, easy option to measure protection against the virus. “People want to know, ‘Am I protected today?’ And there isn’t a tool on the market that can facilitate that answer,” said Charles Mace, a chemist at Tufts University who was not involved in the new study. “This has that potential.” Antibodies float around in the bloodstream, waiting for a foreign invader to make it in. When a virus does, some antibodies that are specific for parts of the virus — neutralizing antibodies — recognize and bind to it, which marks the virus for destruction and prevents it from infecting any cells. The body can naturally develop neutralizing antibodies through exposure to the virus after vaccination or an infection. When scientists want to determine whether those neutralizing antibodies are present in a person, they take blood samples, separate out the serum, and mix it with live SARS-CoV-2 virus. They then incubate that mixture with a human cell line and see how many of those cells die off. Scientists can also use other viruses that have been engineered to make the spike protein and a fluorescent molecule. But both of those methods require live virus, which means training and a biosafety lab, and take about 2 days to run. Researchers have been developing other, faster methods. But by and large, they either require laboratory equipment or are more complicated to manufacture because they use antibodies. The project started when Hojun Li, a hematologist and investigator at the Koch Institute, saw a bone marrow transplant patient at the Dana-Farber Cancer Institute right around the time that the pandemic hit the U.S. hard. But before the operation, they presented with symptoms for Covid-19. At the time, the only option available for doctors to rule out a Covid-19 infection was to do a blood test and examine the antibody levels. It took a week for the results to come back negative and Li to be able to proceed with the bone marrow transplant. “These are usually very urgent, urgent things to do,” Li said. “The longer you wait, the more likely whatever underlying diseases that come back cannot be cured by the bone marrow transplant.” Li and his colleague at the time, Guinevere Connelly, started talking about how they could close that knowledge gap with Covid. Connelly, a co-first author of the study and now a Ph.D. student at Duke University, said they quickly realized “that what would be really helpful would be a neutralizing antibody test where you could see what level of protection you may have against infection from Covid or from SARS-CoV-2.” With collaborators, they learned new techniques to make every component of a test, from producing viral proteins at a larger-scale to cycling through a hundred different buffer formulas. It took them a year to collect enough test samples — repeat blood draws from two people before and after vaccination, and single donations from 93 other individuals — to design and validate the test. The result of their work is a device that looks and works like existing rapid antigen tests, which detect protein from the virus when someone is actively infected. The new test uses a small amount of blood, obtained via a finger prick, that gets mixed with a buffer that contains the portion of the SARS-CoV-2 spike protein that binds to the ACE2 receptor on human cells. Where a typical antigen test has two binding sites — a control line that shows the test is working properly and a test line that lights up when a person is positive for the coronavirus — the new one has three: a control line, a line turns an intense red when a person does not have neutralizing antibodies, and a line that appears when they do. “We also like people to think about positive signals as opposed to reduction of signals because there’s just a general feeling that that’s more reliable,” said Angela Koehler, a bioengineer at MIT who worked on the new test. Indicating both the presence and absence of neutralizing antibodies, the researchers said, allows everyday consumers to snap a picture with their mobile app to know their quantitative antibody titer. “If you have an actual number that you can measure — and neutralizing antibody concentration would be a number that you can actually measure — then you can tell what degree of protection they have and whether or not they might need a subsequent [dose] or a booster sooner rather than later,” said Li. That kind of information could be of interest to a wide range of people, but would be particularly helpful for those who have weaker immune responses, like those Li sees in the clinic. “The vast, vast majority of patients I see get bone marrow transplants,” he added. “Their immune system is just really wiped out.” Basing booster decisions on antibody levels is not too far-fetched an idea. In Australia, for example, antibody testing is used to determine if someone needs a 4th dose of the hepatitis B vaccine. But Julio Delgado, the clinical pathology chief at the University of Utah School of Medicine, is more skeptical that the new test will find use among the broader population. “Even if I get vaccinated, I’m still going to get infected at some point. That happened to me,” said Delgado, who has developed laboratory-based clinical tests that measure levels of antibodies against SARS-CoV-2. “So the understanding about neutralizing antibodies has really become more of a scientific interest, not necessarily a clinical utility.” Some experts said that any use case was overly optimistic because we don’t yet know what antibody levels translate to optimal protection. This is complicated by the fact that different assays calculate titer levels differently. “There is not an understanding about thresholds above or below which people are going to be prone to getting infected, or prone to develop progressive disease, or prone to die,” said Delgado. “Yes, you get a number, but what does that mean?” In part because the new test can be manufactured for less than a dollar a kit, its developers said that is exactly one of the questions their new product can answer. “It’s really the first one that I’ve seen that can answer that question,” said Hadley Sikes, a chemical engineer at MIT who was a senior author of the research. “If there was a study design where you use this tool — you recruit a cohort, say, 5,000 or 10,000 people, you get these neutralizing antibody tests in their homes, and you follow who gets infected and doesn’t get infected — it’s an answer to a question that a lot of people, a lot of doctors, a lot of articles in the press have been raising.” The team behind the test said they are interested in pursuing an emergency use authorization down the road and would want to work with an industry partner or foundation that has experience with regulatory applications. But Li acknowledged that the Food and Drug Administration “has a little bit of hesitancy about anything antibody-related,” but attributed this to tests that measured all antibodies, rather than only SARS-CoV-2 neutralizing antibodies. “A lot of those tests flooded the market and a lot of them, frankly, were very, very poorly managed,” he said. Because antibody levels decline gradually, testing once a month would likely be enough. On the flip side, though, such infrequent testing needs might make it less appealing for commercial players to invest in the studies that would be needed to move the tests from a proof-of-concept to an authorized product. A different approach on the horizon uses a costlier machine called a spectrometer to detect the Covid-fighting antibodies. Cheng-Hao Ko, an engineer at the National Taiwan University of Science and Technology, said this would be “300 to 400 times more accurate” than the rapid test approach. Ko is collaborating with a group at Temple University to seek emergency use authorization from the FDA for that device. That level of accuracy likely wouldn’t be necessary for the general public. “If you have a good indication of low, mid-range, and high level of antibodies, that’s probably good enough for the purpose,” said Jean-François Masson, a biochemist at the Université de Montréal in Canada. Adolfo Garcia-Sastre, a virologist at the Icahn School of Medicine at Mount Sinai, said there’s also a potential to use this type of test as a tool to understand immunity at the population level and help guide public health decisions, for example by routinely testing samples from blood donors. “Surveillance is going to become more important, I hope, in the next year,” he said, “because it gives you an idea of what is the vulnerability.” See also study (August 22, 2022): ttps://doi.org/10.1016/j.crmeth.2022.100273

|
Scooped by
Juan Lama
|
Compared with nasal swabs, saliva tests may better reflect infection deep in the lungs. To the known risk factors for developing severe COVID-19—age, male sex, or any of a series of underlying conditions—a new study adds one more: high levels of the virus in your saliva. Standard COVID-19 tests sample the nasal passage. But several new tests look for SARS-CoV-2, the pandemic coronavirus, in saliva, and the new work finds a striking correlation between high virus levels there and later hospitalization or death. If the results are confirmed, saliva tests could help doctors prioritize which patients in the early stages of the disease should receive medicines that drive down levels of the virus. “I thought it was pretty striking,” says Shane Crotty, a virologist at the La Jolla Institute for Immunology, who was not involved with the research. Crotty notes the results suggest virus levels in saliva reflect viral load deep in the lungs, where the disease does much of its damage in severe cases. “That is a fundamentally valuable insight,” Crotty says. The new work isn’t the first to link the body’s coronavirus load and disease outcome. Several research groups have found a correlation between high viral levels in the nasal passages at the time of a patient’s hospital admission and ultimate disease severity. But other groups have failed to find that same link. The standard test to detect SARS-CoV-2 samples nasal mucus using nasopharyngeal (NP) swabs. The procedure is unpleasant, but it is the customary way to sample respiratory pathogens. In recent months, however, several research groups have developed and received emergency use authorization from the U.S. Food and Drug Administration for tests detecting SARS-CoV-2 in saliva. Yale University researchers were among the first, and the university’s hospitals have been using both saliva and NP swab tests. In both cases, labs analyze the samples using quantitative reverse transcription polymerase chain reaction tests, which can detect genetic material from SARS-CoV-2 and quantify the number of viral particles in each milliliter of sample. Researchers led by Akiko Iwasaki, an immunologist at Yale, compared viral loads in saliva and NP swabs from 154 patients and 109 people without the virus. They divided the patients into groups that had low, medium, and high viral loads as determined by both types of test. Then they compared those results with the severity of symptoms the patients developed later. They found that patients who developed severe disease, were hospitalized, or died were more likely to have had high virus loads in their saliva tests, but not in their NP swabs. Viral load in both saliva and nasal mucus declined over time in patients who recovered, but not in those who died. When Iwasaki and her colleagues reviewed patients’ electronic medical records for markers of disease in the blood, they found that high saliva viral loads correlated with high levels of immune signals such as cytokines and chemokines, nonspecific molecules that ramp up in response to viral infections and have been linked to tissue damage. People with more virus in their saliva also gradually lost certain cells that mount an immune response against viral targets, had lower levels of antibodies targeting the spike protein that the virus uses to enter cells, and were slower to develop the strong immune response needed to knock down the virus in cases where they recovered. The team’s results appeared on 10 January in a preprint that has not been peer reviewed. Iwasaki and her colleagues argue that saliva may be a better predictor of disease outcome than nasal mucus because the latter comes from the upper respiratory tract, whereas severe disease is associated with damage deep in the lungs. “Saliva may better represent what is going on in the lower respiratory tract,” Iwasaki says, because cilia lining the respiratory tract naturally move mucus up from the lungs into the throat, where it mixes with saliva; coughs have the same effect. The results don’t have enough statistical power to reveal how much more likely a person with a high saliva viral load is to develop severe COVID-19, Iwasaki says. She is also eager for other groups to replicate the results, especially because efforts to link high NP swab viral loads with disease progression have had mixed results. If other research confirms the finding, “it would clear away a lot of the fog” around this disease, Crotty says. Monica Gandhi, an infectious disease expert at the University of California, San Francisco, adds that if saliva tests are predictive, they could help doctors identify patients to treat early with either antibodies to reduce viral load or steroids to tamp down overactive nonspecific immune responses. Saliva tests are cheaper and easier than NP tests, but much less widely available. So confirmation of the new results could bolster efforts to make saliva tests more readily available, says Sri Kosuri, CEO of Octant, Inc., a biotech company. “If this study happened in March, we’d be talking about whether we should be doing NP testing at all,” Kosuri says. Preprint available in medRxiv (Jan. 10, 2021): https://doi.org/10.1101/2021.01.04.21249236

|
Scooped by
Juan Lama
|
Rapid antigen tests for the coronavirus are faster, cheaper and more user-friendly than standard diagnostic assays. An assessment now shows that some antigen tests — but not all — can tell with high accuracy who is likely to be most infectious. Antigen-based assays detect specific proteins, or antigens, on the surface of SARS-CoV-2 particles. Christian Drosten at the Charité — University Hospital Berlin and his colleagues analysed the performance of seven commercially available rapid antigen tests. The researchers applied the tests to a range of samples, including dozens of swabs from people who had already tested positive for SARS-CoV-2 or for other respiratory viruses using the gold-standard polymerase chain reaction test (V. M. Corman et al. Preprint at medRxiv https://doi.org/ghj3wt; 2020). The five most sensitive antigen assays detected the presence of SARS-CoV-2 on 95% of the test runs for samples with concentrations of viral genetic material that ranged between the equivalent of 3.4 million and 74 million copies per millilitre of swab. Such high viral levels are observed during the first week of symptoms, when people are likely to spread the virus to others. The findings have not yet been peer reviewed. Study cited available as preprint in medRxiv (November 13, 2020): https://doi.org/10.1101/2020.11.12.20230292

|
Scooped by
Juan Lama
|
Viral levels in people infected with SARS-CoV-2 in a specific town or city could be used to assess whether the epidemic there has passed its peak. A common test for SARS-CoV-2 allows doctors to measure an infected person’s ‘viral load’, an indicator of the amount of virus in their body. James Hay at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues, used modelling to show that the viral loads of a population correlate with the rate of viral spread in that population (J. A. Hay et al. Preprint at medRxiv https://doi.org/ghfm73; 2020). Early in an epidemic, the average infected person has been recently exposed to the virus and therefore has a high viral load. Later in the epidemic, the average infected person has had the virus for longer and has a low viral load. As a result, a snapshot of the viral-load distribution in a random sample of a population can reveal whether cases in that population are on the rise or are declining, the researchers say. They add that their method is less susceptible to biases from changing COVID-testing practices than simply counting daily cases. The findings have not yet been peer reviewed.

|
Scooped by
Juan Lama
|
The most widely used diagnostic test for the virus, called a PCR test, provides a simple yes-no answer to the question of whether a patient is infected. But the results sent to doctors and patients do not include something else the tests reveal: an indication of the amount of virus in the patient’s body, which is a signal of how contagious the person may be. That means many more people than necessary are being required to isolate and submit to contact tracing, and that the true picture of the state of the virus is skewed, according to reporting by Apoorva Mandavilli of The Times. The findings suggest that shifting to faster, less sensitive tests may help communities get a better handle on the virus. “We’ve been using one type of data for everything, and that is just plus or minus — that’s all,” said Dr. Michael Mina, an epidemiologist at the Harvard T.H. Chan School of Public Health. “We’re using that for clinical diagnostics, for public health, for policy decision-making.” The PCR test amplifies genetic matter from the virus in cycles. Large viral loads take fewer cycles to register, while even small amounts of virus — or inactive virus fragments — will register if enough cycles are run. (Dr. Mina thinks the cutoff in cycles should be no more than 30, to limit positives for samples with very little virus.) The number of cycles at which the virus registers is called the cycle threshold, or C.T. The Centers for Disease Control and Prevention said that it was examining the use of C.T. measures “for policy decisions,” and that it would need to collaborate with the Food and Drug Administration and device manufacturers to ensure the measures “can be used properly and with assurance that we know what they mean.” In three sets of testing data that did include C.T. values, compiled by officials in Massachusetts, New York and Nevada, up to 90 percent of positive samples barely carried any virus, a review by The Times found. If that rate applied nationwide, then only about 4,500 of the 45,604 new U.S. cases reported on Thursday would actually require isolation and contact tracing. “It’s just kind of mind-blowing to me that people are not recording the C.T. values from all these tests — that they’re just returning a positive or a negative,” said Angela Rasmussen, a virologist at Columbia University in New York.

|
Scooped by
Juan Lama
|
Few people who have undergone nasopharyngeal swabs for coronavirus testing would describe it as a pleasant experience. The procedure involves sticking a long swab up the nose to collect a sample from the back of the nose and throat, which is then analyzed for SARS-CoV-2 RNA by the reverse-transcription polymerase chain reaction (RT-PCR). Now, researchers reporting in ACS Nano have developed a prototype device that non-invasively detected COVID-19 in the exhaled breath of infected patients. In addition to being uncomfortable, the current gold standard for COVID-19 testing requires RT-PCR, a time-consuming laboratory procedure. Because of backlogs, obtaining a result can take several days. To reduce transmission and mortality rates, healthcare systems need quick, inexpensive and easy-to-use tests. Hossam Haick, Hu Liu, Yueyin Pan and colleagues wanted to develop a nanomaterial-based sensor that could detect COVID-19 in exhaled breath, similar to a breathalyzer test for alcohol intoxication. Previous studies have shown that viruses and the cells they infect emit volatile organic compounds (VOCs) that can be exhaled in the breath. The researchers made an array of gold nanoparticles linked to molecules that are sensitive to various VOCs. When VOCs interact with the molecules on a nanoparticle, the electrical resistance changes. The researchers trained the sensor to detect COVID-19 by using machine learning to compare the pattern of electrical resistance signals obtained from the breath of 49 confirmed COVID-19 patients with those from 58 healthy controls and 33 non-COVID lung infection patients in Wuhan, China. Each study participant blew into the device for 2-3 seconds from a distance of 1¬-2 cm. Once machine learning identified a potential COVID-19 signature, the team tested the accuracy of the device on a subset of participants. In the test set, the device showed 76% accuracy in distinguishing COVID-19 cases from controls and 95% accuracy in discriminating COVID-19 cases from lung infections. The sensor could also distinguish, with 88% accuracy, between sick and recovered COVID-19 patients. Although the test needs to be validated in more patients, it could be useful for screening large populations to determine which individuals need further testing, the researchers say. Original study published in ACS Nano (August 18, 2020): https://doi.org/10.1021/acsnano.0c05657

|
Scooped by
Juan Lama
|
The kit has been granted approval under ‘emergency use’ provisions, and should help to ease testing backlogs in the country. The US drug regulator has granted its first emergency-use approval for a new coronavirus test that takes advantage of the gene-editing technology CRISPR. The US Food and Drug Administration’s (FDA) emergency-use authority allows it to make tests and drugs available faster than usual in a public-health emergency. The new diagnostic kit is based on an approach co-developed by CRISPR pioneer Feng Zhang at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts. It will be used to test for the novel coronavirus behind the ongoing pandemic, SARS-CoV-2, in laboratories that are certified to provide clinical-test results. Although the United States has ramped up testing in the past week — averaging nearly 250,000 tests per day, according to the non-profit organization The COVID Tracking Project — there are test shortages in some places. Widespread use of the new FDA-approved kit could help to alleviate backlogs and increase testing, says Mitchell O’Connell, a biochemist at the University of Rochester in New York, who was not involved in developing the test. But O’Connell cautions that it remains to be seen how well the test performs in real-world conditions, such as hospitals, compared with standard tests. The CRISPR-based diagnostic kit has been developed by Sherlock Biosciences, a biotechnology company also based in Cambridge. It works by programming the CRISPR machinery, which has the ability to home in on certain genetic sequences, to detect a snippet of SARS-CoV-2 genetic material in a nose, mouth or throat swab, or in fluid from the lungs. If the virus’s genetic material is found, a CRISPR enzyme generates a fluorescent glow. The test can return results in about an hour, according to the company....

|
Scooped by
Juan Lama
|
The FDA authorized its first COVID-19 diagnostic test that allows a person to collect a simple saliva sample themselves, without leaving their home, similar to a personal DNA test. The green light expands upon a previous authorization for a test developed by Rutgers University, designed to use a person’s spit instead of a nasal swab. The agency also granted its blessing to LabCorp’s at-home swab test in late April. “Authorizing additional diagnostic tests with the option of at-home sample collection will continue to increase patient access to testing for COVID-19,” FDA Commissioner Stephen Hahn said in a statement. “This provides an additional option for the easy, safe and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site.” “The FDA has authorized more than 80 COVID-19 tests and adding more options for at-home sample collection is an important advancement in diagnostic testing during this public health emergency,” Hahn added. Rutgers’ prescription-only molecular test currently remains as the only diagnostic authorized by the FDA to process saliva samples. After using a collection kit made by Spectrum DNA, the sealed package is then mailed to the New Jersey university’s clinical genomics lab for analysis. Previously, the university said that quickly administered, saliva-based tests could find equally important use in the field—not just in the home—by sparing healthcare workers exposure from collection methods that require them to manually swab a person’s deep nasal cavity....

|
Scooped by
Juan Lama
|
The US Food and Drug Administration announced it has authorized the use of the first rapid diagnostic test that could detect the novel coronavirus in approximately 45 minutes. The authorization was made Friday and tests will begin shipping next week, according to a statement from California-based Cepheid, the company manufacturing the tests. "During this time of increased demand for hospital services, Clinicians urgently need an on-demand diagnostic test for real-time management of patients being evaluated for admission to health-care facilities," said Dr. David Persing, MD, Ph.D., chief medical and technology officer at Cepheid. "An accurate test delivered close to the patient can be transformative -- and help alleviate the pressure that the emergence of the 2019-nCoV outbreak has put on healthcare facilities that need to properly allocate their respiratory isolation resources," Persing added. The announcement of more efficient testing comes as the medical community has been looking to get quicker results to stem the tide of the coronavirus outbreak. Last week, Dr. Rod Hochman, CEO of Providence St. Joseph Health, an organization of 51 hospitals and about 1,000 clinics, described testing capacity in the US as highly deficient. The turnaround time on testing results, he said, had ranged from 24 hours to four days, which he called "unacceptable." If cases of the disease are not identified quickly and community spread continues unchecked, it could soon overwhelm the nation's medical system, just as it did in Wuhan, China, said Dr. Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine. FDA Letter of approval (March 21, 2020):
|

|
Scooped by
Juan Lama
|
GETTING a cough, fever or sore throat at this time of year could be down to any number of things, from a common cold to Covid-19. With a new strain of swine flu being detected in the UK The London Medical Laboratory said its test can be taken at 95 selected pharmacies, drop-in clinics and health stores nationwide. You can find a full list of the places they're sold here. Otherwise, you can also order the test online to be delivered to your home. Dr Narayanan said: "The current strains of flu that are circulating may produce only mild illness in one person but may cause severe symptoms and even prove fatal to others. "This is particularly important in people with pre-existing health conditions and long-term diseases. Similarly, while the RSV virus may only produce chesty cold symptoms in some people, it can severely affect elderly people and children. "All of these viruses, including swine flu, display very similar initial symptoms to the common cold, but these symptoms may quickly escalate." Taking a test to identify which virus is causing your symptoms could bring "peace of mind" to some or ensure "they are not endangering anyone in their family this festive season", the clinical lead claimed. The fact that the UKHSA is still working to ascertain the source of the H1N2 strain spotted in the North Yorkshire resident is another reason people might want to get tested, Dr Narayanan added. Where else can I get these test? The 95 pharmacies and clinics aren't the only place you can take a test telling the difference between the viruses. Online pharmacy Chemist Connect sells a kit by the brand CordX that claims to test for Covid, influenza A and B and RSV. It carries a much lower price tag too - £37.95, along with a delivery charge. Granted, that's still a fair amount pay for a test. Alternatively, you can nab Flowflex Flu Test and Covid Test Bundle at your local Boots. It won't be able to test for RSV - but you'll only have to shell out £4 for the two kits that come in the bundle. You can buy a five-pack of Influenza A/B Rapid Test at Tesco for £12. You can't get a free Covid-19 PCR test any more and rapid lateral flow tests are no longer free for most people. But according to NHS guidance, you might be eligible to get them for free if: - You have a health condition which means you're at high risk of getting seriously ill from Covid
- You work in healthcare settings or in a hospice

|
Scooped by
Juan Lama
|
But the company that created the 30-minute, over-the-counter test has filed for bankruptcy, so the product’s eventual availability to consumers remains unclear. The Food and Drug Administration issued an emergency authorization for the first over-the-counter, at-home combination flu and Covid test on Friday, just two days after the company that makes the test announced that it had filed for bankruptcy protection based, in part, on the agency’s lengthy approval timeline. The single-use test works with a self-collected nasal swab and provides a result in about 30 minutes, according to the F.D.A. The test is meant to be used by people 14 and older, or by an adult collecting a sample from someone age 2 or older. The test’s developer, Lucira Health, based in California’s Bay Area, announced its bankruptcy plan on Wednesday, noting that it had expected its emergency-use authorization for the test in August before the onslaught of the flu season. The company said the agency’s authorization process “became protracted,” and said it had high expenditures as it moved forward with manufacturing the combination tests. Without revenue that the company expected from projected sales of the tests during this year’s flu season, Lucira decided that it would pursue a sale of its business but continue operations to serve customers, according to its news release. The bankruptcy plan was reported earlier in The Wall Street Journal. In a statement issued on Friday, Dr. Jeff Shuren, the director of the F.D.A.’s device division, called the test “a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home.” But even though public health experts and scientists welcomed the test authorization, it remained unclear when such a combined test would be widely available for sale to consumers. And that uncertainty compounded concerns that others have voiced about the Biden administration’s plans to end the coronavirus public health emergency in May, which could complicate access to testing. People with private insurance and those on Medicare, who have been eligible for eight free at-home tests per month, may have to pay out of pocket for the tests once the emergency ends. Erik Engelson, Lucira’s chief executive, said in a statement Friday that the company was “very excited” about the authorization. “I can’t thank our employees and partners enough for seeing this through, and of course, for the F.D.A.’s recognition,” he said. Lucira Health did not immediately respond to questions about its manufacturing capacity or how much the test would cost consumers. The combination test correctly identified 99 percent of negative and 90 percent of positive flu A samples, according to the F.D.A. It also detected 100 percent of the negative and 88 percent of the positive Covid samples. The agency said it expected the company to continue to test on the flu B strain, which was not prevalent this year. The product is a molecular test, which means it detects and amplifies the genetic material of the viruses, as a P.C.R. test does. These tests are generally more sensitive than antigen tests, and at-home molecular tests have been more expensive than rapid antigen tests. The new test will be the first of a series of combination diagnostics in different stages of development that will scan for multiple ailments at once, said Dr. Wilbur Lam, a pediatric hematologist and bioengineer at Emory University who has helped federal officials with test development and validation. “Now we’re in this new era that’s honestly pretty exciting,” Dr. Lam said. “It’s exciting for a health care provider, it’s exciting for the technology developers, and I think exciting for the public because we have this new test. And this is only the beginning.” Through the pandemic, some public health experts have criticized the F.D.A. for being slow to approve at-home Covid tests and the federal government for failing to make the tests more widely available to Americans at little or no cost. Even once at-home tests were approved, fluctuating demand prompted manufacturers to ramp down production, contributing to shortages of rapid tests when the virus came surging back. During the first years of the pandemic, flu activity was unusually low. But last fall, with most pandemic precautions gone, the flu re-emerged in alarming numbers so early in the flu season. Over the last several months, Americans have had to contend with waves of multiple viruses, including influenza, the coronavirus and respiratory syncytial virus, or R.S.V. FDA Press Release (Feb. 24, 2023): https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-over-counter-home-test-detect-both-influenza-and-covid-19-viruses

|
Scooped by
Juan Lama
|
The Omicron variant is characterised by more than 50 distinct mutations, the majority of which are located in the spike protein. The implications of these mutations for disease transmission, tissue tropism and diagnostic testing are still to be determined. We evaluated the relative performance of saliva and mid-turbinate swabs as RT-PCR samples for the Delta and Omicron variants. The positive percent agreement (PPA) of saliva swabs and mid-turbinate swabs to a composite standard was 71% (95% CI: 53-84%) and 100% (95% CI: 89-100%), respectively, for the Delta variant. However, for the Omicron variant saliva and mid-turbinate swabs had a 100% (95% CI: 90-100%) and 86% (95% CI: 71-94%) PPA, respectively. This finding supports ex-vivo data of altered tissue tropism from other labs for the Omicron variant. Reassessment of the diagnostic testing standard-of-care may be required as the Omicron variant becomes the dominant variant worldwide. Preprint available at medRxiv (Dec. 24, 2021): https://doi.org/10.1101/2021.12.22.21268246

|
Scooped by
Juan Lama
|
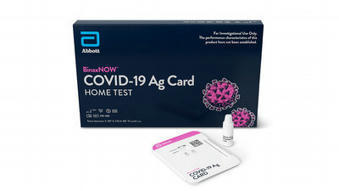
- First at-home, virtually guided service to bring rapid, reliable and affordable testing into the home where the result is delivered in minutes - Abbott and eMed™ expect to deliver and administer 30 million BinaxNOW™ tests in the first quarter of 2021, with an additional 90 million in the second quarter - $25 cost for the test and service is the lowest available for at-home testing, which supports the home user to ensure a successful testing process - BinaxNOW is a highly portable, easy-to-use, 15-minute antigen test with no instrument that detects the virus when people are most infectious, which is critically important in slowing the spread of the virus - Service uses Abbott's complementary NAVICA™ app to enable the testing process and display authenticated BinaxNOW test results verified by a trained guide - and supports consumer confidence in testing at home ABBOTT PARK, Ill.,, Dec. 16, 2020 /PRNewswire/ -- Abbott (NYSE: ABT) announced today that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for virtually guided at-home use of its BinaxNOW™ COVID-19 Ag Card rapid test for detection of COVID-19 infection. Abbott is bringing BinaxNOW, the most affordable and widely distributed rapid test into the home, where the result is delivered in minutes without the need to send it out for processing. The $25 cost for the test and service is the lowest currently available for at-home testing. To facilitate the delivery of the BinaxNOW test to the home and the guided collection and testing process, Abbott has partnered with digital health solutions provider eMed™. This agreement furthers Abbott's vision of making as many tests as possible available in a variety of different settings to improve accessibility, support consumer confidence in testing at home, and help people start returning to living their daily lives with more normalcy. Abbott and eMed expect to deliver and administer 30 million BinaxNOW at-home tests in the first quarter of 2021, with an additional 90 million in the second quarter. Scale is critical to provide broad access to people who need testing and Abbott has scale for all its eight COVID-19 lab and rapid tests. In fact, since launching BinaxNOW in August, Abbott has ramped up capacity to 50 million tests a month in its U.S. facilities that are currently being distributed through the federal government and is expanding further so even more people have access to the tests. "As the pandemic has evolved, the need for rapid testing has only grown. Unfortunately, we're still hearing that many people can't access testing as quickly as they need it," said Robert B. Ford, Abbott's president and chief executive officer. "That's why Abbott is bringing our rapid BinaxNOW test and NAVICA platform into homes through this partnership with eMed, which allows us to maintain the integrity of the testing process, get even closer to people who need testing and help provide the confidence we need to help get back to living with a bit more normalcy." The eMed service offering costs $25 a test, and eMed takes care of determining eligibility, the guided self-collection process, public health reporting requirements and gets people their results through their NAVICA app in a matter of minutes. The eMed offering could also remove some of the obstacles that individuals, governments and organizations face when seeking to provide or perform testing. These include mobility issues for people with disabilities or who may not have a reliable form of transportation, inconvenient testing windows for people who work non-traditional hours or have childcare or eldercare responsibilities, and discouragingly long lines at testing centers. FDA Release Dec. 16, 2020: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-new-authorization-binaxnow-covid-19-ag-card-home-test

|
Scooped by
Juan Lama
|
An artificial intelligence model can detect people who are asymptomatic with Covid-19, through cellphone-recorded coughs. The work was led by Brian Subirana and colleagues at the MIT Auto-ID Lab. Asymptomatic people who are infected with Covid-19 exhibit, by definition, no discernible physical symptoms of the disease. They are thus less likely to seek out testing for the virus, and could unknowingly spread the infection to others. But it seems those who are asymptomatic may not be entirely free of changes wrought by the virus. MIT researchers have now found that people who are asymptomatic may differ from healthy individuals in the way that they cough. These differences are not decipherable to the human ear. But it turns out that they can be picked up by artificial intelligence. In a paper published recently in the IEEE Journal of Engineering in Medicine and Biology, the team reports on an AI model that distinguishes asymptomatic people from healthy individuals through forced-cough recordings, which people voluntarily submitted through web browsers and devices such as cellphones and laptops. The researchers trained the model on tens of thousands of samples of coughs, as well as spoken words. When they fed the model new cough recordings, it accurately identified 98.5 percent of coughs from people who were confirmed to have Covid-19, including 100 percent of coughs from asymptomatics — who reported they did not have symptoms but had tested positive for the virus. The team is working on incorporating the model into a user-friendly app, which if FDA-approved and adopted on a large scale could potentially be a free, convenient, noninvasive prescreening tool to identify people who are likely to be asymptomatic for Covid-19. A user could log in daily, cough into their phone, and instantly get information on whether they might be infected and therefore should confirm with a formal test. “The effective implementation of this group diagnostic tool could diminish the spread of the pandemic if everyone uses it before going to a classroom, a factory, or a restaurant,” says co-author Brian Subirana, a research scientist in MIT’s Auto-ID Laboratory. Subirana’s co-authors are Jordi Laguarta and Ferran Hueto, of MIT’s Auto-ID Laboratory.. Study cited published in IEEE Journal of Engineering in Medicine and Biology (Sept.30, 2020): https://www.embs.org/ojemb/articles/covid-19-artificial-intelligence-diagnosis-using-only-cough-recordings/

|
Scooped by
Juan Lama
|
CRISPR-based approach is much faster than current diagnostics, but does have some limitation. CRISPR diagnostics are just one way researchers are trying to speed coronavirus testing. The new test is the fastest CRISPR-based diagnostic yet. In May, for example, two teams reported creating CRISPR-based coronavirus tests that could detect the virus in about an hour, much faster than the 24 hours needed for conventional coronavirus diagnostic tests. CRISPR tests work by identifying a sequence of RNA—about 20 RNA bases long—that is unique to SARS-CoV-2. They do so by creating a “guide” RNA that is complementary to the target RNA sequence and, thus, will bind to it in solution. When the guide binds to its target, the CRISPR tool’s Cas13 “scissors” enzyme turns on and cuts apart any nearby single-stranded RNA. These cuts release a separately introduced fluorescent particle in the test solution. When the sample is then hit with a burst of laser light, the released fluorescent particles light up, signaling the presence of the virus. These initial CRISPR tests, however, required researchers to first amplify any potential viral RNA before running it through the diagnostic to increase their odds of spotting a signal. That added complexity, cost, and time, and put a strain on scarce chemical reagents. Now, researchers led by Jennifer Doudna, who won a share of this year’s Nobel Prize in Chemistry yesterday for her co-discovery of CRISPR, report creating a novel CRISPR diagnostic that doesn’t amplify coronavirus RNA. Instead, Doudna and her colleagues spent months testing hundreds of guide RNAs to find multiple guides that work in tandem to increase the sensitivity of the test. In a new preprint, the researchers report that with a single guide RNA, they could detect as few as 100,000 viruses per microliter of solution. And if they add a second guide RNA, they can detect as few as 100 viruses per microliter. That’s still not as good as the conventional coronavirus diagnostic setup, which uses expensive lab-based machines to track the virus down to one virus per microliter, says Melanie Ott, a virologist at UC San Francisco who helped lead the project with Doudna. However, she says, the new setup was able to accurately identify a batch of five positive clinical samples with perfect accuracy in just 5 minutes per test, whereas the standard test can take 1 day or more to return results. The new test has another key advantage, Wilson says: quantifying a sample’s amount of virus. When standard coronavirus tests amplify the virus’ genetic material in order to detect it, this changes the amount of genetic material present—and thus wipes out any chance of precisely quantifying just how much virus is in the sample. By contrast, Ott’s and Doudna’s team found that the strength of the fluorescent signal was proportional to the amount of virus in their sample. That revealed not just whether a sample was positive, but also how much virus a patient had. That information can help doctors tailor treatment decisions to each patient’s condition, Wilson says. Doudna and Ott say they and their colleagues are now working to validate their test setup and are looking into how to commercialize it. Preprint available at medRxiv (Sept. 30, 2020): https://doi.org/10.1101/2020.09.28.20201947

|
Scooped by
Juan Lama
|
One of the keys to bringing a viral outbreak under control is rapid detection and diagnosis, which depend on the availability of fast, low-cost, easy-to-use tests that don't require labs or expensive equipment to process them. Scientists at the Broad Institute of MIT and Harvard and collaborators in the United States, Nigeria, and Sierra Leone have now validated such tests for Ebola and Lassa—two of the deadliest and most transmissible human viruses—in settings with limited infrastructure. The work appears in Nature Communications. The diagnostic tests use the CRISPR-based SHERLOCK assay to detect low levels of virus in patient samples and generate either a fluorescent readout or a result on a paper strip. The test can be tailored to detect specific viruses from certain regions, requires only a simple heat block and basic supplies to run, costs less than US$1 per sample, could be used on saliva or urine—eliminating the need for blood draws—and can return results in less than an hour. The tests also use a rapid chemical and heat treatment called HUDSON to inactive the virus in patient samples. HUDSON makes the patient samples safer for clinical staff to handle in a low-tech environment, and eliminates the need to extract a virus's genetic material from the samples before analyzing. The research team was led at Broad by Kayla Barnes, an NIH Fogarty K fellow at the Harvard School of Public Health and Broad Institute, Anna Lachenauer, a medical student at Stanford University School of Medicine, and institute member Pardis Sabeti, a professor at Harvard University and investigator with the Howard Hughes Medical Institute. To showcase SHERLOCK's field utility, team members led by Christian Happi at Redeemer's University in Nigeria deployed a Lassa-specific assay during a recent Lassa fever outbreak—the first use of SHERLOCK in a low/middle income country. The team also compared the diagnostic against a standard RT-qPCR assay for Lassa. Collaborators at Kenema Government Hospital in Sierra Leone and at the US Army Medical Research Institute of Infectious Diseases benchmarked an Ebola-specific version of the SHERLOCK assay, using samples collected during the 2014-16 outbreak in Sierra Leone and more recent outbreaks in the Democratic Republic of the Congo. The NIH Integrated Research Facility also validated HUDSON's ability to heat-inactivate Ebola virus in their BL4 facilities, further establishing the safety and efficiency of this step. The SHERLOCK assays performed as consistently as, or better than, other diagnostics in these validations—demonstrating the platform's potential for clinical use in the future in resource-limited areas. The team also developed a mobile phone app called HandLens, spearheaded by Andres Colubri, assistant professor in the Bioinformatics and Integrative Biology program at the University of Massachusetts Medical School, that can read and immediately report paper strip SHERLOCK results. The tool can aid in situations where the paper strip gives a faint signal that is challenging for a clinician to interpret. This app can be adapted for use on any smartphone or tablet, according to the researchers, allowing a clear, unbiased diagnostic readout. Published in Nature Communications (August 17, 2020): https://doi.org/10.1038/s41467-020-17994-9

|
Scooped by
Juan Lama
|
The program allows people in the Seattle area to easily take a coronavirus test at home. Researchers say such testing is essential for future monitoring of the virus. An innovative coronavirus testing program in the Seattle area — promoted by the billionaire Bill Gates and local public health officials as a way of conducting wider surveillance on the invisible spread of the virus — has been ordered by the federal government to stop its work pending additional reviews. The program involved sending home test kits to both healthy and sick people in the hope of conducting the kind of widespread monitoring that could help communities safely reopen from lockdowns. Researchers and public health authorities already had tested thousands of samples, finding dozens of previously undetected cases. But the program, a partnership between research groups and the Seattle and King County public health department that had been operating under authorization from the state, was notified this week that it now needs approval directly from the federal government. Officials with the Food and Drug Administration told the partnership to cease its testing and reporting until the agency grants further approval. “Please discontinue patient testing and return of diagnostic results to patients until proper authorization is obtained,” the F.D.A. wrote in a memo. The delay is the latest evidence of how a splintered national effort to develop, distribute and ramp up testing has left federal regulators struggling to keep up. Amid concerns about the reliability of a burgeoning number of coronavirus antibody tests — which check whether someone may have previously had the virus — the F.D.A. responded last week by ordering companies to submit data proving the tests’ accuracy. But the Seattle program does not test for antibodies and has wide backing, including from public health leaders, the Fred Hutchinson Cancer Research Center and Mr. Gates, whose foundation has been deeply involved in fighting the pandemic. The Centers for Disease Control and Prevention also provided an in-person technical adviser to the project.

|
Scooped by
Juan Lama
|
This Viewpoint discusses the 2 most common categories of testing to diagnose SARS-CoV-2—real-time PCR to identify viral RNA and serological diagnosis of IgG and IgM antibodies to assess immune response—and estimates time intervals for test positivity by specimen source to help clinician. Thus far, the most commonly used and reliable test for diagnosis of COVID-19 has been the RT-PCR test performed using nasopharyngeal swabs or other upper respiratory tract specimens, including throat swab or, more recently, saliva. A variety of RNA gene targets are used by different manufacturers, with most tests targeting 1 or more of the envelope (env), nucleocapsid (N), spike (S), RNA-dependent RNA polymerase (RdRp), and ORF1 genes. The sensitivities of the tests to individual genes are comparable according to comparison studies except the RdRp-SARSr (Charité) primer probe, which has a slightly lower sensitivity likely due to a mismatch in the reverse primer. In most individuals with symptomatic COVID-19 infection, viral RNA in the nasopharyngeal swab as measured by the cycle threshold (Ct) becomes detectable as early as day 1 of symptoms and peaks within the first week of symptom onset. The Ct is the number of replication cycles required to produce a fluorescent signal, with lower Ct values representing higher viral RNA loads. A Ct value less than 40 is clinically reported as PCR positive. This positivity starts to decline by week 3 and subsequently becomes undetectable. However, the Ct values obtained in severely ill hospitalized patients are lower than the Ct values of mild cases, and PCR positivity may persist beyond 3 weeks after illness onset when most mild cases will yield a negative result.However, a “positive” PCR result reflects only the detection of viral RNA and does not necessarily indicate presence of viable virus.... Published in JAMA (May 6, 2020): https://doi.org/10.1001/jama.2020.8259

|
Scooped by
Juan Lama
|
Abbott on Friday announced it received approval for a test that is capable of delivering positive results of the coronavirus in as little as five minutes, and it will begin making those tests available to health care providers next week. The Food and Drug Administration issued emergency use authorization for the point-of-care test on Friday, the company said in a statement. The test can detect negative results in 13 minutes. The company said it plans to ramp up manufacturing so it can deliver 50,000 tests per day. “The COVID-19 pandemic will be fought on multiple fronts, and a portable molecular test that offers results in minutes adds to the broad range of diagnostic solutions needed to combat this virus,” Abbott Chief Operating Officer Robert Ford said in a statement. This is the second Abbott test for the coronavirus to be launched. Between the two, the company expects to produce about 5 million tests per month, the company said in a statement. The Lake Bluff, Illinois, company makes diagnostics, medical devices, nutritionals and medicines. A challenge might be that clinicians, due to the personal protective equipment shortage, might not feel safe administering these tests to patients, especially those with respiratory symptoms.

|
Scooped by
Juan Lama
|
Roche received a green light from the FDA for emergency use of its high-volume coronavirus diagnostic, the first commercially developed test to do so. The agency said it approved the test within 24 hours of receiving the application. The fully automated test is expected to substantially boost testing capacity in the U.S. in the coming days. Previously, the FDA authorized preapproved but validated testing for the novel coronavirus at specific, certified “high complexity” laboratories. Now, this latest Emergency Use Authorization enables clinicians to run the Roche test on the company’s cobas 6800/8800 molecular testing systems, widely available at hospitals and labs throughout the country. It is also available in European countries accepting the CE mark. In addition to the one-day approval, the FDA said it did not object to Roche pre-shipping its COVID-19 tests to laboratories ahead of time, so they could be used immediately following the authorization. “We have been encouraging test developers to come to the FDA and work with us,” said the agency’s device and diagnostic center director, Jeff Shuren. “Since the beginning of this outbreak, more than 60 developers have sought our assistance with development and validation of tests they plan to bring through the Emergency Use Authorization process,” Shuren addedd. “Additionally, more than 30 laboratories have notified us they are testing or intend to begin testing soon under our new policy for laboratory developed tests for this emergency.” Previously, the FDA granted emergency authorizations to diagnostic panels developed by the CDC and the New York State Department of Health. The agency also granted the state health department the authority to allow certain labs to proceed with testing without any federal approval or regulatory submissions. Those labs would instead interact solely with New York State health officials.
|



 Your new post is loading...
Your new post is loading...