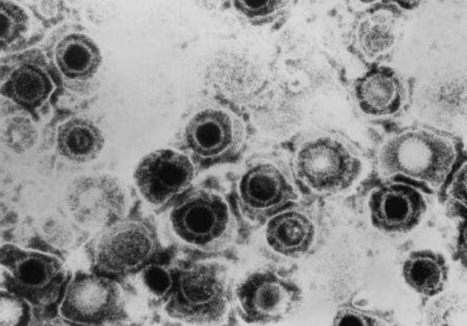
The herpes simplex virus, commonly known as the cold sore virus, is a devious microbe. It enters the body through regions lined with mucous membranes—mouth, nose and genitals—but quickly establishes lifelong viral hideouts inside nerve cells. After initial infection, the virus lurks dormant only to be reawakened periodically to cause outbreaks marked by the eruption of cold sores or blisters. In a handful of people, the consequences of viral re-awaking can be devastating, including blindness and brain inflammation. Antiviral medications can prevent recurrent outbreaks, but they are not always effective, so for decades, researchers have sought a solution that would quiet the virus for good. Now, using human fibroblast cells infected with herpes simplex virus (HSV), researchers at Harvard Medical School have successfully used CRISPR-Cas9 gene editing to disrupt not only actively replicating virus but also the far-harder to reach dormant pools of the virus, demonstrating a possible strategy for achieving permanent viral control. The team's findings are described Dec. 2 in eLife.
"This is an exciting first step—one that suggests it is possible to permanently silence lifelong infections—but much more work remains to be done," said study lead investigator David Knipe, the Higgins Professor of Microbiology and Molecular Genetics in the Blavatnik Institute at Harvard Medical School. Notably, the research represents the first successful instance of disrupting latent viral reservoirs through gene editing. Latent reservoirs are notoriously impervious to antiviral medications and have also proven hard to gene-edit. The experiments also identify the mechanisms by which actively replicating virus becomes uniquely vulnerable to gene editing. These very mechanisms may also explain why latent forms of the virus are less amenable to this technique.
Specifically, the experiments reveal that the DNA of an actively replicating virus is more exposed to the Cas9 enzyme—the molecular scissors in the CRISPR-Cas9 gene-editing system. This is because actively replicating viruses have fewer protective histones that wrap around their DNA to shield it. "The absence of protective histones makes the DNA more accessible and easier to cut, so it's essentially identified HSV's Achilles heel," Knipe said. The new findings offer a model system for using gene editing in a localized way to disrupt active replication in specific sites. However, Knipe cautions, the arch-challenge of delivering gene-editing therapy to neurons—where the virus hides and enters a state of dormancy—remains to be solved, Knipe added...
Published in eLife (December 2, 2019):
https://doi.org/10.7554/eLife.51662



 Your new post is loading...
Your new post is loading...




https://farmaciadimagrante.com/
https://farmaciadimagrante.com/Prodotto/acquista-mysimba-online/
https://farmaciadimagrante.com/Prodotto/acquista-mounjaro-online/
https://farmaciadimagrante.com/Prodotto/acquista-victoza-online/
https://farmaciadimagrante.com/Prodotto/acquistare-saxenda-6mg-ml-online/
https://farmaciadimagrante.com/Prodotto/acquista-ozempic-online/
https://farmaciadimagrante.com/Prodotto/acquista-wegovy-online/
https://farmaciadimagrante.com/Prodotto/acquista-nembutal-in-polvere-online/
https://farmaciadimagrante.com/Prodotto/acquista-online-nembutal-solution/
https://farmaciadimagrante.com/Prodotto/acquista-ketamina-hcl-500mg-10ml-in-linea/
https://farmaciadimagrante.com/Prodotto/acquistare-fentanyl-in-polvere-online/
https://farmaciadimagrante.com/Prodotto/acquistare-fentanyl-online/
https://farmaciadimagrante.com/Prodotto/acquista-cristallo-mdma-online/
https://farmaciadimagrante.com/Prodotto/acquista-ativan-online/
https://farmaciadimagrante.com/Prodotto/acquista-botox-online/
https://farmaciadimagrante.com/Prodotto/acquista-cerotti-al-fentanil/
https://farmaciadimagrante.com/Prodotto/acquista-codeina-linctus-online/
https://farmaciadimagrante.com/Prodotto/acquista-codeina-online/
https://farmaciadimagrante.com/Prodotto/acquista-demerol-online/
https://farmaciadimagrante.com/Prodotto/acquista-depalgo-online/
https://farmaciadimagrante.com/Prodotto/acquista-diazepam-online/
https://farmaciadimagrante.com/Prodotto/acquista-instanyl-online/
https://farmaciadimagrante.com/Prodotto/acquista-l-ritalin-online/
https://farmaciadimagrante.com/Prodotto/acquista-metadone/
https://farmaciadimagrante.com/Prodotto/acquista-opana-online/
https://farmaciadimagrante.com/Prodotto/acquista-stilnox-online/
https://farmaciadimagrante.com/Prodotto/acquista-suboxone-8mg/
https://farmaciadimagrante.com/Prodotto/acquista-subutex-online/
https://farmaciadimagrante.com/Prodotto/acquista-vicodin-online/
https://farmaciadimagrante.com/Prodotto/acquista-vyvanse-online/
https://farmaciadimagrante.com/Prodotto/acquista-xanax-2mg/
https://farmaciadimagrante.com/Prodotto/acquistare-rohypnol-2mg/
https://farmaciadimagrante.com/Prodotto/acquistare-sibutramina-online/
https://farmaciadimagrante.com/Prodotto/efedrina-hcl-in-polvere/
https://farmaciadimagrante.com/Prodotto/ephedrine-hcl-30mg/
https://farmaciadimagrante.com/Prodotto/sciroppo-di-metadone/
https://farmaciadimagrante.com/Prodotto/tramadolo-hcl-200mg/
https://farmaciadimagrante.com/Prodotto/acquista-adipex-online/
https://farmaciadimagrante.com/Prodotto/acquista-adderall-30mg/
https://farmaciadimagrante.com/Prodotto/acquista-oxycontin-online/
https://farmaciadimagrante.com/Prodotto/acquista-ossicodone-online/
https://farmaciadimagrante.com/Prodotto/acquista-phentermine-online/
https://farmaciadimagrante.com/Prodotto/acquista-ambien/
https://farmaciadimagrante.com/Prodotto/acquista-percocet-online/
https://farmaciadimagrante.com/Prodotto/acquistare-buprenorfina-8mg-2mg/
https://farmaciadimagrante.com/Prodotto/a-215-ossicodone-actavis/
https://farmaciadimagrante.com/Prodotto/acquista-eroina-bianca/
<a href="https://farmaciadimagrante.com/Prodotto/acquista-mysimba-online/">acquista-mysimba-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-mounjaro-online/">acquista-mounjaro-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-victoza-online/">acquista-victoza-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquistare-saxenda-6mg-ml-online/">acquistare-saxenda-6mg-ml-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-ozempic-online/">acquista-ozempic-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-wegovy-online/">acquista-wegovy-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-nembutal-in-polvere-online/">acquista-nembutal-in-polvere-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-online-nembutal-solution/">acquista-online-nembutal-solution</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-ketamina-hcl-500mg-10ml-in-linea/">acquista-ketamina-hcl-500mg-10ml-in-linea</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquistare-fentanyl-in-polvere-online/">acquistare-fentanyl-in-polvere-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquistare-fentanyl-online/">acquistare-fentanyl-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-ativan-online/">acquista-ativan-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-botox-online/">acquista-botox-online</a></a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-cerotti-al-fentanil/">acquista-cerotti-al-fentanil</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-codeina-linctus-online/">acquista-codeina-linctus-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-codeina-online/">acquista-codeina-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-demerol-online/">acquista-demerol-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-depalgo-online/">acquista-depalgo-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-diazepam-online/">acquista-diazepam-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-instanyl-online/">acquista-instanyl-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-l-ritalin-online/">acquista-l-ritalin-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-metadone/">acquista-metadone</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-opana-online/">acquista-opana-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-stilnox-online/">acquista-stilnox-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-suboxone-8mg/">acquista-suboxone-8mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-subutex-online/">acquista-subutex-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-vicodin-online/">acquista-vicodin-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-vyvanse-online/">acquista-vyvanse-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-xanax-2mg/">acquista-xanax-2mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquistare-rohypnol-2mg/">acquistare-rohypnol-2mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquistare-sibutramina-online/">acquistare-sibutramina-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/efedrina-hcl-in-polvere/">efedrina-hcl-in-polvere</a>;
<a href="https://farmaciadimagrante.com/Prodotto/ephedrine-hcl-30mg/">ephedrine-hcl-30mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/sciroppo-di-metadone/">sciroppo-di-metadone</a>;
<a href="https://farmaciadimagrante.com/Prodotto/tramadolo-hcl-200mg/">tramadolo-hcl-200mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-adipex-online/">acquista-adipex-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-adderall-30mg/">acquista-adderall-30mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-oxycontin-online/">acquista-oxycontin-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-ossicodone-online/">acquista-ossicodone-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-phentermine-online/">acquista-phentermine-online</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-ambien/">acquista-ambien</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-percocet-online/">acquistare-buprenorfina-8mg-2mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquistare-buprenorfina-8mg-2mg/">acquistare-buprenorfina-8mg-2mg</a>;
<a href="https://farmaciadimagrante.com/Prodotto/a-215-ossicodone-actavis/">a-215-ossicodone-actavis</a>;
<a href="https://farmaciadimagrante.com/Prodotto/acquista-eroina-bianca/">acquista-eroina-bianca</a>;
https://globaalapotheek.com/product/koop-adderall-online/
https://globaalapotheek.com/product/efedrine-hcl-poeder-kopen/
https://globaalapotheek.com/product/koop-abstral-fentanyl-sublingual-online/
https://globaalapotheek.com/product/koop-actavis-hoestsiroop-online/
https://globaalapotheek.com/product/koop-adipex-online/
https://globaalapotheek.com/product/koop-ambien-online/
https://globaalapotheek.com/product/koop-ativan-online/
https://globaalapotheek.com/product/koop-botox-online/
https://globaalapotheek.com/product/koop-bromazepam-online/
https://globaalapotheek.com/product/koop-buprenorfine-online/
https://globaalapotheek.com/product/koop-desoxyn-online/
https://globaalapotheek.com/product/koop-dexedrine-online/
https://globaalapotheek.com/product/koop-diamorfine-online/
https://globaalapotheek.com/product/koop-dianabol-online/
https://globaalapotheek.com/product/koop-dysport-online/
https://globaalapotheek.com/product/koop-ecstasy-online/
https://globaalapotheek.com/product/koop-efedrine-hcl-online/
https://globaalapotheek.com/product/koop-endocet-online/
https://globaalapotheek.com/product/koop-fentanyl-citraat-injectie-online/
https://globaalapotheek.com/product/koop-fentanyl-pleisters-actavis/
https://globaalapotheek.com/product/koop-fentanyl-pleisters-mylan/
https://globaalapotheek.com/product/koop-fentanyl-sandoz-5x-100mcg/
https://globaalapotheek.com/product/koop-fentanyl-sandoz-5x-375mcg/
https://globaalapotheek.com/product/koop-focalin-xr-online/
https://globaalapotheek.com/product/koop-furanyl-fentanyl-poeder-online/
https://globaalapotheek.com/product/koop-humatrope-online/
https://globaalapotheek.com/product/koop-hydromorfoon-online/
https://globaalapotheek.com/product/koop-klonopin-online/
https://globaalapotheek.com/product/koop-ksalol-xanax-online/
https://globaalapotheek.com/product/koop-methadon-online/
https://globaalapotheek.com/product/koop-modafinil-online/
https://globaalapotheek.com/product/koop-morfine-sulfaat-200mg-online/
https://globaalapotheek.com/product/koop-morfine-sulfaat-30mg-online/
https://globaalapotheek.com/product/koop-morfine-sulfaat-60mg-online/
https://globaalapotheek.com/product/koop-neurobloc-online/
https://globaalapotheek.com/product/koop-norco-online/
https://globaalapotheek.com/product/koop-oramorph-online/
https://globaalapotheek.com/product/koop-oxycodon-80mg-online/
https://globaalapotheek.com/product/koop-oxycontin-online/
https://globaalapotheek.com/product/koop-oxymorfoon-online/
https://globaalapotheek.com/product/koop-percocet-online/
https://globaalapotheek.com/product/koop-quaalude-online/
https://globaalapotheek.com/product/koop-restoril-30mg-online/
https://globaalapotheek.com/product/koop-ritalin-online/
https://globaalapotheek.com/product/koop-roxicodone-online/
https://globaalapotheek.com/product/koop-soma-online/
https://globaalapotheek.com/product/koop-stilnox-online/
https://globaalapotheek.com/product/koop-suboxone-online/
https://globaalapotheek.com/product/koop-subutex-online/
https://globaalapotheek.com/product/koop-tramadol-online/
https://globaalapotheek.com/product/koop-triazolam-halcion-online/
https://globaalapotheek.com/product/koop-valium-online/
https://globaalapotheek.com/product/koop-vicodin-online/
https://globaalapotheek.com/product/koop-vyvanse-50mg-online/
https://globaalapotheek.com/product/koop-vyvanse-70mg-online/
https://globaalapotheek.com/product/koop-xanax-online/
https://globaalapotheek.com/product/koop-xls-max-online/
https://globaalapotheek.com/product/koop-zaleplon-online/
https://globaalapotheek.com/product/koop-zopiclon-online/
https://globaalapotheek.com/product/morfine-kopen/
https://globaalapotheek.com/product/morfine-injectie-kopen/
https://globaalapotheek.com/product/oxycodon-40mg-kopen-sandoz/
https://globaalapotheek.com/product/oxycodon-80mg-kopen-sandoz/
https://globaalapotheek.com/product/phentermine-online-kopen/
https://globaalapotheek.com/product/vyvanse-kopen/
<a href="https://globaalapotheek.com/product/efedrine-hcl-poeder-kopen/">efedrine-hcl-poeder-kopen</a>;
<a href="https://globaalapotheek.com/product/koop-abstral-fentanyl-sublingual-online/">koop-abstral-fentanyl-sublingual-online</a>;
<a href="https://globaalapotheek.com/product/koop-actavis-hoestsiroop-online/">koop-actavis-hoestsiroop-online</a>;
<a href="https://globaalapotheek.com/product/koop-adderall-online/">koop-adderall-online</a>;
<a href="https://globaalapotheek.com/product/koop-adipex-online/">koop-adipex-online</a>;
<a href="https://globaalapotheek.com/product/koop-ambien-online/">koop-ambien-online</a>;
<a href="https://globaalapotheek.com/product/koop-ativan-online/">koop-ativan-online</a>;
<a href="https://globaalapotheek.com/product/koop-botox-online/">koop-botox-online</a>;
<a href="https://globaalapotheek.com/product/koop-bromazepam-online/">koop-bromazepam-online</a>;
<a href="https://globaalapotheek.com/product/koop-buprenorfine-online/">koop-buprenorfine-online</a>;
<a href="https://globaalapotheek.com/product/koop-desoxyn-online/">koop-desoxyn-online</a>;
<a href="https://globaalapotheek.com/product/koop-dexedrine-online/">koop-dexedrine-online</a>;
<a href="https://globaalapotheek.com/product/koop-diamorfine-online/">koop-diamorfine-online</a>;
<a href="https://globaalapotheek.com/product/koop-dianabol-online/">koop-dianabol-online</a>;
<a href="https://globaalapotheek.com/product/koop-dysport-online/">koop-dysport-online</a>;
<a href="https://globaalapotheek.com/product/koop-ecstasy-online/">koop-ecstasy-online</a>;
<a href="https://globaalapotheek.com/product/koop-efedrine-hcl-online/">koop-efedrine-hcl-online</a>;
<a href="https://globaalapotheek.com/product/koop-endocet-online/">koop-endocet-online</a>;
<a href="https://globaalapotheek.com/product/koop-fentanyl-citraat-injectie-online/">koop-fentanyl-citraat-injectie-online</a>;
<a href="https://globaalapotheek.com/product/koop-fentanyl-pleisters-actavis/">koop-fentanyl-pleisters-actavis</a>;
<a href="https://globaalapotheek.com/product/koop-fentanyl-pleisters-mylan/">koop-fentanyl-pleisters-mylan</a>;
<a href="https://globaalapotheek.com/product/koop-fentanyl-sandoz-5x-100mcg/">koop-fentanyl-sandoz-5x-100mcg</a>;
<a href="https://globaalapotheek.com/product/koop-fentanyl-sandoz-5x-375mcg/">koop-fentanyl-sandoz-5x-375mcg</a>;
<a href="https://globaalapotheek.com/product/koop-focalin-xr-online/">koop-focalin-xr-online</a>;
<a href="https://globaalapotheek.com/product/koop-furanyl-fentanyl-poeder-online/">koop-furanyl-fentanyl-poeder-online</a>;
<a href="https://globaalapotheek.com/product/koop-humatrope-online/">koop-humatrope-online</a>;
<a href="https://globaalapotheek.com/product/koop-hydromorfoon-online/">koop-hydromorfoon-online</a>;
<a href="https://globaalapotheek.com/product/koop-klonopin-online/">koop-klonopin-online</a>;
<a href="https://globaalapotheek.com/product/koop-ksalol-xanax-online/">koop-ksalol-xanax-online</a>;
<a href="https://globaalapotheek.com/product/koop-methadon-online/">koop-methadon-online</a>;
<a href="https://globaalapotheek.com/product/koop-modafinil-online/">koop-modafinil-online</a>;
<a href="https://globaalapotheek.com/product/koop-morfine-sulfaat-200mg-online/">koop-morfine-sulfaat-200mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-morfine-sulfaat-30mg-online/">koop-morfine-sulfaat-30mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-morfine-sulfaat-60mg-online/">koop-morfine-sulfaat-60mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-neurobloc-online/">koop-neurobloc-online</a>;
<a href="https://globaalapotheek.com/product/koop-norco-online/">koop-norco-online</a>;
<a href="https://globaalapotheek.com/product/koop-oramorph-online/">koop-oramorph-online</a>;
<a href="https://globaalapotheek.com/product/koop-oxycodon-80mg-online/">koop-oxycodon-80mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-oxycontin-online/">koop-oxycontin-online</a>;
<a href="https://globaalapotheek.com/product/koop-oxymorfoon-online/">koop-oxymorfoon-online</a>;
<a href="https://globaalapotheek.com/product/koop-percocet-online/">koop-percocet-online</a>;
<a href="https://globaalapotheek.com/product/koop-quaalude-online/">koop-quaalude-online</a>;
<a href="https://globaalapotheek.com/product/koop-restoril-30mg-online/">koop-restoril-30mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-ritalin-online/">koop-ritalin-online</a>;
<a href="https://globaalapotheek.com/product/koop-roxicodone-online/">koop-roxicodone-online</a>;
<a href="https://globaalapotheek.com/product/koop-soma-online/">koop-soma-online</a>;
<a href="https://globaalapotheek.com/product/koop-stilnox-online/">koop-stilnox-online</a>;
<a href="https://globaalapotheek.com/product/koop-suboxone-online/">koop-suboxone-online</a>;
<a href="https://globaalapotheek.com/product/koop-subutex-online/">koop-subutex-online</a>;
<a href="https://globaalapotheek.com/product/koop-tramadol-online/">koop-tramadol-online</a>;
<a href="https://globaalapotheek.com/product/koop-triazolam-halcion-online/">koop-triazolam-halcion-online</a>;
<a href="https://globaalapotheek.com/product/koop-valium-online/">koop-valium-online</a>;
<a href="https://globaalapotheek.com/product/koop-vicodin-online/">koop-vicodin-online</a>;
<a href="https://globaalapotheek.com/product/koop-vyvanse-50mg-online/">koop-vyvanse-50mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-vyvanse-70mg-online/">koop-vyvanse-70mg-online</a>;
<a href="https://globaalapotheek.com/product/koop-xanax-online/">koop-xanax-online</a>;
<a href="https://globaalapotheek.com/product/koop-xls-max-online/">koop-xls-max-online</a>;
<a href="https://globaalapotheek.com/product/koop-zaleplon-online/">koop-zaleplon-online</a>;
<a href="https://globaalapotheek.com/product/koop-zopiclon-online/">koop-zopiclon-online</a>;
<a href="https://globaalapotheek.com/product/morfine-kopen/">morfine-kopen</a>;
<a href="https://globaalapotheek.com/product/morfine-injectie-kopen/">morfine-injectie-kopen</a>;
<a href="https://globaalapotheek.com/product/oxycodon-40mg-kopen-sandoz/">oxycodon-40mg-kopen-sandoz</a>;
<a href="https://globaalapotheek.com/product/oxycodon-80mg-kopen-sandoz/">oxycodon-80mg-kopen-sandoz</a>;
<a href="https://globaalapotheek.com/product/phentermine-online-kopen/">phentermine-online-kopen</a>;
<a href="https://globaalapotheek.com/product/vyvanse-kopen/">vyvanse-kopen</a>;
https://perderepesoefedrina.com/
https://perderepesoefedrina.com/Prodotto/acquista-ossicodone-online/
https://perderepesoefedrina.com/Prodotto/acquista-oxycontin-online/
https://perderepesoefedrina.com/Prodotto/acquista-percocet-online/
https://perderepesoefedrina.com/Prodotto/acquista-phentermine-online/
https://perderepesoefedrina.com/Prodotto/acquista-eroina-bianca/
https://perderepesoefedrina.com/Prodotto/a-215-ossicodone-actavis/
https://perderepesoefedrina.com/Prodotto/acquista-adderall-30mg/
https://perderepesoefedrina.com/Prodotto/acquista-adipex-online/
https://perderepesoefedrina.com/Prodotto/acquista-adma-online/
https://perderepesoefedrina.com/Prodotto/acquista-ambien/
https://perderepesoefedrina.com/Prodotto/acquista-ativan-online/
https://perderepesoefedrina.com/Prodotto/acquista-botox-online/
https://perderepesoefedrina.com/Prodotto/acquista-cerotti-al-fentanil/
https://perderepesoefedrina.com/Prodotto/acquista-codeina-linctus-online/
https://perderepesoefedrina.com/Prodotto/acquista-codeina-online/
https://perderepesoefedrina.com/Prodotto/acquista-demerol-online/
https://perderepesoefedrina.com/Prodotto/acquista-depalgo-online/
https://perderepesoefedrina.com/Prodotto/acquista-diazepam-online/
https://perderepesoefedrina.com/Prodotto/acquista-dilaudid-8mg/
https://perderepesoefedrina.com/Prodotto/acquista-endocet-online/
https://perderepesoefedrina.com/Prodotto/acquista-green-xanax/
https://perderepesoefedrina.com/Prodotto/acquista-hydrocodone-online/
https://perderepesoefedrina.com/Prodotto/acquista-instanyl-online/
https://perderepesoefedrina.com/Prodotto/acquista-l-ritalin-online/
https://perderepesoefedrina.com/Prodotto/acquista-metadone/
https://perderepesoefedrina.com/Prodotto/acquista-morfina-solfato/
https://perderepesoefedrina.com/Prodotto/acquista-opana-online/
https://perderepesoefedrina.com/Prodotto/acquista-roxicodone-30mg/
https://perderepesoefedrina.com/Prodotto/acquista-stilnox-online/
https://perderepesoefedrina.com/Prodotto/acquista-suboxone-8mg/
https://perderepesoefedrina.com/Prodotto/acquista-subutex-online/
https://perderepesoefedrina.com/Prodotto/acquista-vicodin-online/
https://perderepesoefedrina.com/Prodotto/acquista-vyvanse-online/
https://perderepesoefedrina.com/Prodotto/acquista-xanax-2mg/
https://perderepesoefedrina.com/Prodotto/acquistare-dapoxetina-online/
https://perderepesoefedrina.com/Prodotto/acquistare-rohypnol-2mg/
https://perderepesoefedrina.com/Prodotto/acquistare-sibutramina-online/
https://perderepesoefedrina.com/Prodotto/efedrina-hcl-in-polvere/
https://perderepesoefedrina.com/Prodotto/ephedrine-hcl-30mg/
https://perderepesoefedrina.com/Prodotto/sciroppo-di-metadone/
https://perderepesoefedrina.com/Prodotto/tramadolo-hcl-200mg/
https://perderepesoefedrina.com/Prodotto/acquista-cristallo-mdma-online/