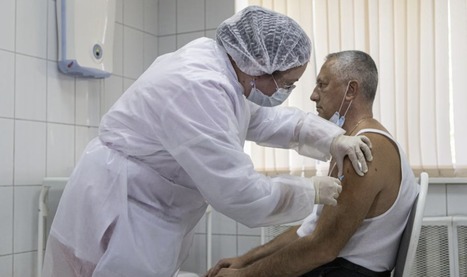
The vaccine has only been tested in a small-scale trial with 100 volunteers, results of which are yet to be made available. Russia has granted regulatory approval to a second Covid-19 vaccine—even though the drug has yet to begin large scale Phase-III trials—two months after it greenlit another vaccine that experts expressed caution over due to lack of adequate safety and efficacy data.
The second vaccine was announced by Russian President Vladimir Putin at a government meeting on Wednesday, Reuters reported. The peptide-based drug, named EpiVacCorona, has been developed by the Vector Institute in Siberia. A placebo-controlled trial had 100 volunteers, between the ages of 18 and 60, receive the shot in the city of Novosibirsk. Results of those early-stage trials are yet to be published and large-scale Phase-III trials, which help establish safety and efficacy, have not yet begun. Putin said that Russia will increase the production of both the vaccines that have been approved by the government and plans to work with “foreign partners” to promote the vaccine abroad.
In August, Russia became the first country to grant regulatory approval to a Covid-19 vaccine, despite limited testing. That vaccine, named-Sputnik-V, was developed by Moscow’s Gamaleya Institute. Scientists expressed concern that without proper trial data, there was no way of knowing if the vaccine was actually effective or safe. The Russian President, however, claimed that the drug was both safe and effective noting that it had already been administered to one of his daughters. Hundreds of people who work in a place that puts them at high risk for getting infected with the coronavirus have been inoculated with the Sputnik-V shot. The vaccine though is not yet in general use. When Sputnik-V was announced, former FDA Commissioner Scott Gotlieb said that Russia was “certainly not” ahead of the U.S. on Covid-19 vaccine development, noting that the U.S. would not allow mass distribution of a drug had been only tested on a few hundred patients at most...



 Your new post is loading...
Your new post is loading...