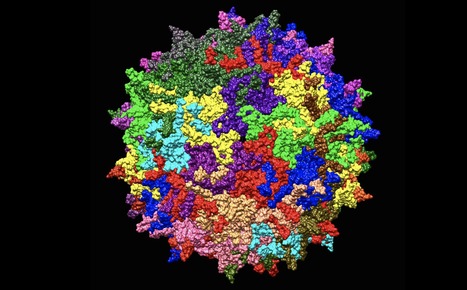
Researchers have identified a novel cellular entry factor for adeno-associated virus vector (AAV) types -- the most commonly used viral vectors for in vivo gene therapy. The researchers identified that GPR108, a G protein-coupled receptor, served as a molecular 'lock' to the cell. The discovery could one day enable scientists to better direct AAV gene transfers to specific tissues. The researchers identified that GPR108, a G protein-coupled receptor, served as a molecular 'lock' to the cell. GPR108 is required for most AAVs, including those used in approved gene therapies, to gain access to the cell. As gaining cellular access is a critical step in delivering gene therapy, this discovery may provide a crucial piece of information that could one day enable scientists to better explain, predict, and ultimately, direct AAV gene transfers to specific tissues. The study was recently published in Molecular Therapy.
"For years we have known that AAV gene transfer is highly effective, but we have yet to learn how that is achieved and why some AAV types function differently than others," said senior study author Luk Vandenberghe, PhD, Director of the Grousbeck Gene Therapy Center at Mass. Eye and Ear and Associate Professor of Ophthalmology at Harvard Medical School. "We identified a molecular 'lock' to the cell that allows AAV vectors carrying the appropriate 'key' to gain access to the cell. This finding may enable scientists to better direct AAV gene transfers to targeted cell tissues, in order to treat specific genetic diseases."
Multiple AAV types are in clinical trials for diseases affecting the eye, muscles, and neurons. Luxturna™ and Zolgensma™, both recently approved by the U.S. Food and Drug Administration, are AAV gene therapy products for a form of blindness and neuromuscular disease. Yet, the exact mechanism by which this novel class of medicine accomplishes gene transfer has remained poorly understood.
Published in Molecular Therapy (November 13, 2019):



 Your new post is loading...
Your new post is loading...