Your new post is loading...

|
Scooped by
Juan Lama
|
Background: Subvariants of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) omicron XBB-lineage have the potential to escape immunity provided by prior infection or vaccination. For Covid-19 immunizations beginning in the Fall 2023, the U.S. FDA has recommended updating to a monovalent omicron XBB.1.5-containing vaccine. Methods: In this ongoing, phase 2/3 study participants were randomized 1:1 to receive 50-µg doses of mRNA-1273.815 monovalent (50-µg omicron XBB.1.5 spike mRNA) or mRNA-1273.231 bivalent (25-µg omicron XBB.1.5 and 25-µg omicron BA.4/BA.5 spike mRNAs) vaccines, administered as 5th doses, to adults who previously received a primary series and 3rd dose of an original mRNA coronavirus disease 2019 (Covid-19) vaccine, and a 4th dose of a bivalent (omicron BA.4/BA.5 and original SARS-CoV-2) vaccine. Interim safety and immunogenicity data 15 days post-vaccination are presented. Results: In April 2023, participants received mRNA-1273.815 (n=50) and mRNA-1273.231 (n=51). The median intervals from the prior dose of BA.4/BA.5-containing bivalent vaccine were 8.2 and 8.3 months for the mRNA-1273.815 and mRNA-1273.231 groups, respectively. Both vaccines increased neutralizing antibody (nAb) geometric mean titers against all variants tested at day 15 post-booster nAb compared to pre-booster levels. Geometric mean fold-rises from pre-booster titers after the monovalent booster were numerically higher against XBB.1.5, XBB.1.16 and SARS-CoV-2 (D614G) than those of the bivalent booster and were comparable against BA.4/BA.5 and BQ1.1 variants for both vaccines. The monovalent vaccine also elicited nAb responses against omicron XBB.2.3.2, EG.5.1, FL.1.5.1 and BA.2.86 that were similar to those against XBB.1.5 in a subset (n=20) of participants. The occurrence of solicited adverse reactions and unsolicited adverse events were overall similar to those previously reported for the original mRNA-1273 50-µg and omicron BA.4/BA.5-containing bivalent mRNA-1273 vaccines. Conclusion: In this interim analysis, XBB.1.5-containing monovalent and bivalent vaccines elicited potent neutralizing responses against variants of the omicron XBB-lineage (XBB.1.5, XBB.1.6, XBB.2.3.2, EG.5.1, and FL.1.5.1) as well as the recently emerged BA.2.86 variant. The safety profile of the XBB.1.5-containing vaccine was consistent with those of prior vaccines. These results overall indicate that the XBB.1.5-containing mRNA-1273.815 vaccine has the potential to provide protection against these emerging variants and support the Covid-19 vaccine update in 2023-2024 to a monovalent XBB.1.5-containing vaccine. Preprint avilable in medRxiv (Sept. 7, 2023): https://doi.org/10.1101/2023.08.22.23293434

|
Scooped by
Juan Lama
|
Pfizer Inc said on Thursday its updated COVID-19 shot, which is being tested against emerging variants, showed neutralizing activity against the "Eris" subvariant in a study conducted on mice.

|
Scooped by
Juan Lama
|
An experimental mRNA cancer vaccine developed by Moderna Inc and Merck & Co cut the risk of death or recurrence of the most deadly skin cancer by 44% compared with Merck's immunotherapy Keytruda alone, U.S. researchers reported at a medical meeting on Sunday.

|
Scooped by
Juan Lama
|
Not long after Moderna kicked off its COVID-19 vaccine launch, questions started swirling around the origins of the company's mRNA technology and the intellectual property rights to its vaccines. In Moderna's earnings release Thursday, the company said it recently paid the National Institute of Allergy and Infectious Diseases (NIAID) a $400 million "catch-up payment" under a new royalty-bearing license agreement between the parties. The payment is part of a license agreement between Moderna and NIAID inked late last year. With the deal, Moderna is paying the U.S. government to access “certain patent rights concerning stabilizing prefusion coronavirus spike proteins,” Moderna Chief Financial Officer Jamie Mock said on a conference call Thursday. Going forward, Moderna agreed to pay NIAID “low single-digit royalties” on COVID-19 vaccine sales, Mock added. This agreement does not put Moderna out of the woods on the patent litigation front. Even after this deal, the vaccine maker is fighting with the U.S. National Institutes of Health over the origins of the core technology in the vaccine, The New York Times points out. Within the industry, the company is facing patent suits from mRNA rivals Pfizer and BioNTech, plus a separate case from Arbutus and Roivant’s Genevant Sciences. One case between Pfizer and Moderna is heading to trial in London next April, Reuters reported last week. Moderna pulled down around $36 billion in COVID-19 vaccine sales across 2021 and 2022, its two big launch years. While the $400 million payment represents only around 1% of the company's total COVID-19 vaccine sales over that span, the lump-sum nature of the "catch-up payment" drove up Moderna's fourth quarter's costs. The payment was a "key driver" in pushing the company's cost of sales—as a proportion of total sales—to 39% in the fourth quarter, Mock said. That compared with 14% during the same period in 2021. Aside from the license payment, Moderna also suffered financially from demand declines last year. For all of 2022, the company recorded around $2.8 billion in charges related to slouching demand. That figure included a $1.3 billion charge for inventory write-downs plus $725 million for contract cancellations. The company also paid $776 million related to unused manufacturing capacity and CDMO charges.

|
Scooped by
Juan Lama
|
An experimental personalized mRNA vaccine in combination with the immunotherapy Keytruda reduced the risk of recurrence or death from melanoma in patients who had already had surgery, Moderna and Merck said Tuesday. The randomized trial included 157 patients with stage 3 or stage 4 melanoma who had already had surgery. Some patients received nine doses of the experimental cancer vaccine made by Moderna and the immunotherapy made by Merck every three weeks for about a year, and some received only the immunotherapy. Treatment with the experimental vaccine in combination with the immunotherapy reduced the risk of cancer recurrence or death by 44% compared with the immunotherapy alone, the companies said. The preliminary results of a Phase 2b trial were shared in a news release and have not been peer-reviewed or published. The companies said they will publish the full data in the future and share results at an upcoming conference. The companies said they will initiate a Phase 3 study in melanoma patients next year, and will study additional tumor types. “Today’s results are highly encouraging for the field of cancer treatment. mRNA has been transformative for COVID-19, and now, for the first time ever, we have demonstrated the potential for mRNA to have an impact on outcomes in a randomized clinical trial in melanoma,” Stéphane Bancel, chief executive officer for Moderna, said in a news release. Moderna is the maker of one of the mRNA Covid-19 vaccines used in the United States. Moderna and Merck said serious treatment-related adverse events occurred in 14.4% of patients who received the vaccine and immunotherapy in the trial, and in 10% of patients who received only the immunotherapy. Keytruda has some known risks for serious side effects, the companies said. Moderna’s experimental cancer vaccine, mRNA-4157/V940, is designed to prime patients’ immune system to generate a response to their specific tumors. Merck’s Keytruda, which is already used in the treatment of melanoma, stimulates the immune system to attack tumors. According to the American Cancer Society, melanoma accounts for about 1% of all skin cancers, but it causes a majority of skin cancer deaths. It estimates that in 2022, about 100,000 new melanomas will be diagnosed, and more than 7,600 people will die from melanoma. Press release (Dec. 13, 2022): https://investors.modernatx.com/news/news-details/2022/Moderna-and-Merck-Announce-mRNA-4157V940-an-Investigational-Personalized-mRNA-Cancer-Vaccine-in-Combination-with-KEYTRUDAR-pembrolizumab-Met-Primary-Efficacy-Endpoint-in-Phase-2b-KEYNOTE-942-Trial/default.aspx

|
Scooped by
Juan Lama
|
The emergence of the highly divergent SARS-CoV-2 Omicron variant has jeopardized the efficacy of vaccines based on the ancestral spike. The bivalent COVID-19 mRNA booster vaccine within the United States is comprised of the ancestral and the Omicron BA.5 spike. Since its approval and distribution, additional Omicron subvariants have been identified with key mutations within the spike protein receptor binding domain that are predicted to escape vaccine sera. Of particular concern is the R346T mutation which has arisen in multiple subvariants, including BA.2.75.2 and BQ.1.1. Using a live virus neutralization assay, we evaluated serum samples from individuals who had received either one or two monovalent boosters or the bivalent booster to determine neutralizing activity against wild-type (WA1/2020) virus and Omicron subvariants BA.1, BA.5, BA.2.75.2, and BQ.1.1. In the one monovalent booster cohort, relative to WA1/2020, we observed a reduction in neutralization titers of 9-15-fold against BA.1 and BA.5 and 28-39-fold against BA.2.75.2 and BQ.1.1. In the BA.5-containing bivalent booster cohort, the neutralizing activity improved against all the Omicron subvariants. Relative to WA1/2020, we observed a reduction in neutralization titers of 3.7- and 4-fold against BA.1 and BA.5, respectively, and 11.5- and 21-fold against BA.2.75.2 and BQ.1.1, respectively. These data suggest that the bivalent mRNA booster vaccine broadens humoral immunity against the Omicron subvariants. Preprint in bioRxiv (Nov. 1, 2022): https://doi.org/10.1101/2022.10.31.514636

|
Scooped by
Juan Lama
|
The SARS-CoV-2 Omicron variant and its numerous sub-lineages have exhibited a striking ability to evade humoral immune responses induced by prior vaccination or infection. The Food and Drug Administration (FDA) has recently granted Emergency Use Authorizations (EUAs) to new bivalent formulations of the original Moderna and Pfizer mRNA SARS-CoV-2 vaccines that target both the ancestral strain as well as the Omicron BA.4/BA.5 variant. Despite their widespread use as a vaccine boost, little is known about the antibody responses induced in humans. Here, we collected sera from several clinical cohorts: individuals after three or four doses of the original monovalent mRNA vaccines, individuals receiving the new bivalent vaccines as a fourth dose, and individuals with BA.4/BA.5 breakthrough infection following mRNA vaccination. Using pseudovirus neutralization assays, these sera were tested for neutralization against an ancestral SARS-CoV-2 strain, several Omicron sub-lineages, and several related sarbecoviruses. At ~3-5 weeks post booster shot, individuals who received a fourth vaccine dose with a bivalent mRNA vaccine targeting BA.4/BA.5 had similar neutralizing antibody titers as those receiving a fourth monovalent mRNA vaccine against all SARS-CoV-2 variants tested, including BA.4/BA.5. Those who received a fourth monovalent vaccine dose had a slightly higher neutralizing antibody titers than those who received the bivalent vaccine against three related sarbecoviruses: SARS-CoV, GD-Pangolin, and WIV1. When given as a fourth dose, a bivalent mRNA vaccine targeting Omicron BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not induce superior neutralizing antibody responses in humans, at the time period tested, compared to the original monovalent vaccine formulation. Preprint available in bioRxiv (Oct. 24, 2022): https://www.biorxiv.org/content/10.1101/2022.10.22.513349v1 https://doi.org/10.1101/2022.10.22.513349

|
Scooped by
Juan Lama
|
WASHINGTON — The Food and Drug Administration on Wednesday authorized the first redesign of coronavirus vaccines since they were rolled out in late 2020, setting up millions of Americans to receive new booster doses targeting Omicron subvariants as soon as next week. The agency cleared two options aimed at the BA.5 variant of Omicron that is now dominant: one made by Pfizer and its German partner BioNTech for use in people as young as 12, and the other by Moderna, for those 18 and older. The doses can be given at least two months since people last received a booster dose or completed their initial series of vaccinations. Biden administration officials have argued that even as researchers work to understand how protective the new shots might be, inoculating Americans again in the coming weeks could help curb the persistently high number of infections and deaths. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose,” Dr. Robert M. Califf, the F.D.A. commissioner, said in a statement on Wednesday. He added that the vaccine would “provide better protection against currently circulating variants.” An average of about 90,000 infections and 475 deaths are recorded every day around the United States, almost three years into a pandemic that has killed more than a million Americans and driven a historic drop in life expectancy. But there are also hopeful signs. Even with high case counts, fewer than 40,000 people are currently hospitalized with the virus, a decrease of 10 percent since early August and far fewer than during the Delta-driven surge last summer or the Omicron-fueled wave last winter. Deaths have also remained somewhat flat in recent weeks, a sign that vaccines are helping to prevent the worst outcomes of Covid-19. Ample evidence suggests that many Americans will hold back from getting the updated boosters, either because they are weary of the pandemic or may not feel urgency about an additional dose. With each new shot offered, there are fewer takers. As more companies bring workers back to offices and students return to campuses this fall, persuading Americans to get the updated booster shots will be a major challenge for the administration. The companies produced the retooled shots with extraordinary speed, a testament to the mRNA technology that Pfizer and Moderna have harnessed since the early months of the coronavirus outbreak. The Food and Drug Administration advised companies only two months ago on the formulation that they should adopt for the new vaccines. By later this week, millions of those doses will be delivered to states. The tight timeline meant that the companies went to federal regulators this summer with more limited data on the redesigned boosters than a traditional review process would call for, generating some controversy. Regulators authorized the vaccine without results from human trials, which have just started. Federal officials argue that because the coronavirus is evolving so quickly, human trials would be out of date before they can deliver results that could be used in the F.D.A.’s authorization decision. Instead, they are relying on the results of mouse trials and earlier human trials by Pfizer and Moderna of reformulations aimed at previous versions of the virus. The Biden administration is casting the shots as a standard upgrade that Americans should embrace ahead of potential surges in cases in the winter, like the flu shot, which is reconfigured every year to target more current versions of the influenza virus. The boosters are arriving during a period when the White House has been largely quiet on the pandemic. President Biden has rarely commented on the coronavirus in recent months, even after he tested positive in July. The White House no longer holds regular news briefings on the federal pandemic response, as it did in the first year of the administration — a reflection of the weariness of many Americans in keeping up with Covid precautions. “Covid-19 is the third leading cause of death in the United States. And it’s as if we’ve just accepted that that is going to be the case,” said Mercedes Carnethon, an epidemiologist at Northwestern University’s Feinberg School of Medicine. “I really hope as many people as possible will seek the updated booster so we can protect those who will have a terrible outcome.” Vaccinations remain the cornerstone of the federal government’s Covid strategy, even with tests and treatments widely available. The Biden administration has ordered over 170 million doses for the fall campaign, and officials do not expect shortages when they are rolled out. “If it’s freezing cold out and you have children, you’re going to dress them warmly. This is the concept here,” said Dr. Paul G. Auwaerter, the clinical director of the infectious diseases division at the Johns Hopkins University School of Medicine. “You’ll want to head into the respiratory season with a virus that we know has surprised us with a booster.” Exactly how protective the shots might be is unknown, Dr. Auwaerter said. He pointed to the modest increases in neutralizing antibodies that the companies found in vaccines they tested this year that targeted the original form of Omicron. How antibody levels would translate to protection with the new vaccines was unclear, he added. Experts warned against trying to choose Moderna’s shot over Pfizer’s or vice versa; with research in humans just beginning, scientists are months from knowing whether one brand offers better protection than the other. Many Americans have recently been infected with variants in the Omicron family and have some protection from their bouts with the virus, a development that federal agencies may take into account when recommending how the new shots are used. An advisory committee to the Centers for Disease Control and Prevention is scheduled to meet this week to make recommendations. “For most people, the risk of death is so low at this point, because they’ve gotten infected or vaccinated, or more likely both,” said Dr. Gregory A. Poland, a professor of medicine and infectious diseases and the director of the Vaccine Research Group at the Mayo Clinic. Dr. Poland, who has advised Moderna, Pfizer and White House officials on coronavirus vaccines, said updating booster shots the way the Food and Drug Administration did on Wednesday amounted to a “chase your tail” strategy, tweaking the design incrementally to try to keep up with a fast-changing virus. The new boosters, he said, could potentially save some lives among the elderly and those with immune deficiencies. But they were unlikely to make as substantial an impact with the rest of the population.

|
Scooped by
Juan Lama
|
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched an early-stage clinical trial evaluating an investigational vaccine to prevent infection with Nipah virus. The experimental vaccine is manufactured by Moderna, Inc., Cambridge, Massachusetts, and was developed in collaboration with NIAID’s Vaccine Research Center. It is based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the Phase 1 clinical study, which is being conducted at the NIH Clinical Center in Bethesda, Maryland. Nipah virus infection is a zoonotic disease, meaning that it is spread between animals and people. Fruit bats are the natural host for the virus. The first known Nipah outbreak occurred in 1998 in Malaysia and Singapore and resulted in 265 human cases and 105 deaths, and caused significant economic damage to the swine industry there. Since 1999, outbreaks have occurred annually in Asia, primarily in Bangladesh and India. The virus can cause mild-to-severe disease rapidly progressing from respiratory infection symptoms to encephalitis (brain swelling) leading to coma or death. An estimated 40% to 75% of people infected with Nipah virus die. Although most cases are transmitted via animals, person-to-person transmission can occur. Currently, there is no licensed vaccine or treatment for Nipah virus infection. “Nipah virus poses a considerable pandemic threat because it mutates relatively easily, causes disease in a wide range of mammals, can transmit from person-to-person, and kills a large percentage of the people it infects,” said NIAID Director Anthony S. Fauci, M.D. “The need for a preventive Nipah virus vaccine is significant.” NIAID’s Pandemic Preparedness Plan, published earlier this year, established a framework to study viruses of pandemic potential and prioritize research on prototype pathogens, such as Nipah virus. This is the first clinical trial using the prototype pathogen approach since the plan’s publication. The experimental mRNA-1215 Nipah virus vaccine will be tested in a dose-escalation clinical trial to evaluate its safety, tolerability, and ability to generate an immune response in 40 healthy adults ages 18 to 60 years. Specifically, four groups of 10 participants each will receive two doses of the investigational vaccine via injection in the shoulder muscle four or 12 weeks apart. Group one (10 participants) will receive two 25-microgram (mcg) injections; group two will receive two 50-mcg injections; and group three will receive two 100-mcg injections, each four weeks apart. The vaccine dose for the fourth group of participants will be determined based on an interim analysis of the results from the three previous groups. The fourth group will receive two injections 12 weeks apart. Study participants will be evaluated through clinical observation and blood collection at specified times throughout the study and will be followed by clinical study staff through 52 weeks following their final vaccination. For more information about the clinical trial, visit ClinicalTrials.gov using the study identifier NCT05398796. NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing, and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.

|
Scooped by
Juan Lama
|
A significantly stronger immune response against Omicron is seen, but it remains unclear how an updated vaccine will fare against future versions of the virus. Moderna released preliminary results on Wednesday on an updated coronavirus vaccine that targets the Omicron variant, calling it “our lead candidate” to serve as a U.S. booster shot in the fall. The firm’s researchers tested a booster dose combining the original vaccine with one that specifically targeted Omicron, the variant that became dominant last winter. They found that among those with no evidence of prior coronavirus infection, the combination produced 1.75 times the level of neutralizing antibodies against Omicron as the existing Moderna vaccine did alone. While those results may seem encouraging on their face, many experts worry that the virus is evolving so quickly that it is outpacing the ability to modify vaccines, at least as long as the United States relies on human clinical trials for results. Moderna’s new findings, from a clinical trial involving 814 volunteers, indicate that the updated vaccine produced a significantly stronger immune response against Omicron than the existing vaccine a month after the booster shot was given. The booster shots followed three earlier doses of Moderna’s vaccine. But Omicron has been spawning subvariants for months, and some vaccine experts say that what matters now is how well a new booster formulation would protect against the latest subvariants, BA.4 and BA.5, not Omicron itself. First detected in South Africa early this year, those two subvariants now account for 13 percent of new cases in the United States, and are spreading fast. By some estimates, within a month they could outcompete two other Omicron subvariants, BA.2 and BA.2.12.1, which are dominant at present. Moderna did not release any data on how the updated vaccine worked against BA.4 or BA.5. In a presentation Wednesday morning, Dr. Stephen Hoge, the firm’s president, said that researchers were still gathering data on those and other subvariants. But he said that a very small sample, together with isolated other studies, suggested that the levels of neutralizing antibodies triggered by the updated vaccine were two to threefold lower against the BA.4 and BA.5 subvariants, compared to those triggered against Omicron. But he said those levels were “still a very comfortable place,” a view echoed by at least one federal health official who has reviewed the data. Moderna officials said they could not say yet whether the reconfigured vaccine will offer more lasting protection than the existing one, but they were hopeful it would, based on earlier findings from a study of a vaccine reconfigured against a different variant, released in April. The newest subvariants seem to spread even more quickly than earlier versions of Omicron, and may be better at dodging the immune system’s defenses. It is unclear whether they cause more severe disease. Dr. Anthony S. Fauci, the chief medical adviser to the White House, said in an interview on Tuesday that South Africa, where BA.4 and BA.5 have been widespread, had “seen a slight uptick in hospitalizations, but I.C.U. utilization and deaths are really staying stably low.” In any case, given how fast the virus is mutating, some vaccine experts say it makes more sense to target its most recent versions, rather than forms of the virus that have already been overtaken, or soon will be. The problem is that Moderna and Pfizer — the maker of the other main coronavirus vaccine in the United States — do not have enough time now to run more human clinical trials and still manufacture shots before the fall, when the Biden administration is hoping to be able to offer an updated vaccine to counter what public health experts predict will be a winter surge. That might force regulators to choose updated vaccines based on data from laboratory tests and trials involving mice or other animals, rather than robust human trials. It is also possible that another new variant or subvariant of concern will appear by the fall, further complicating the picture. Outside advisers to the Food and Drug Administration are scheduled to meet June 28 to discuss which vaccine formulation would work best as a fall booster; vaccine manufacturers have said they would need to start production soon “Of course, the final decision is always left to the F.D.A.,” Dr. Fauci said. “But what the F.D.A. will likely do is keep as many irons on the fire as they possibly can. And they may need to revert to alternative pathways of decision, which are laboratory data and possible animal data.” Asked if Americans would accept a booster formulation without lengthy human trials, he said, “People who really are very concerned about protecting themselves will.” John Moore, a virologist at Weill Cornell Medicine in New York, said many health care professionals would be comfortable at this point in the pandemic switching to a different model for coronavirus vaccine development, more like the one used to modify the flu vaccine every year. Federal health officials said that the composition of the annual flu vaccine is changed to meet new variants with minimal new human tests. Moderna’s trial of the vaccine targeting Omicron began in late February. The average age of the participants was 57. All volunteers had received three shots of Moderna’s existing vaccine — two shots, followed by a booster dose given an average of eight months after the second shot. About four and a half months after that first booster, 377 volunteers received a second booster with the existing vaccine, while 437 received the booster designed to work against Omicron. The updated booster produced a stronger immune response among both those who had previously been infected with the virus and those who had not. Overall, those who got the updated booster had a 59 percent higher level of neutralizing antibodies than those who got the existing booster, according to data released by Moderna. Antibodies are the body’s first line of defense in warding off infection from the coronavirus. Other immune responses that also defend against Covid-19 were not measured; those tests are far more complex and time-consuming to conduct. Dr. Paul Burton, Moderna’s chief medical officer, described the results as highly encouraging. “We really feel like this is a sort of fundamental turning point in our fight against this virus — that we can adapt to a variant,” he said. But Dr. Moore said that a less than twofold increase in neutralizing antibodies over the existing vaccine is “only a modest benefit.” “Does that justify switching vaccine composition, given the cost and the logistics and everything else that’s involved?” he asked. “That’s what the argument is going to be about.” Pfizer and BioNTech, its German partner, are also testing an Omicron-specific vaccine and are expected to release their results soon. In April, Moderna released preliminary results on a vaccine retooled to attack the Beta variant, which was first detected in late 2020. That version of the vaccine, the firm said, triggered a stronger immune response than the initial formulation not only against Beta, but also against the Delta and Omicron variants. Although Moderna officials said the added protection against Omicron persisted for six months, they said that they expected an Omicron-specific vaccine would be a better candidate.

|
Scooped by
Juan Lama
|
BACKGROUND Vaccination of children to prevent coronavirus disease 2019 (Covid-19) is an urgent public health need. The safety, immunogenicity, and efficacy of the mRNA-1273 vaccine in children 6 to 11 years of age are unknown. METHODS Part 1 of this ongoing phase 2–3 trial was open label for dose selection; part 2 was an observer-blinded, placebo-controlled expansion evaluation of the selected dose. In part 2, we randomly assigned children (6 to 11 years of age) in a 3:1 ratio to receive two injections of mRNA-1273 (50 μg each) or placebo, administered 28 days apart. The primary objectives were evaluation of the safety of the vaccine in children and the noninferiority of the immune response in these children to that in young adults (18 to 25 years of age) in a related phase 3 trial. Secondary objectives included determination of the incidences of confirmed Covid-19 and severe acute respiratory syndrome coronavirus 2 infection, regardless of symptoms. Interim analysis results are reported. RESULTS In part 1 of the trial, 751 children received 50-μg or 100-μg injections of the mRNA-1273 vaccine, and on the basis of safety and immunogenicity results, the 50-μg dose level was selected for part 2. In part 2 of the trial, 4016 children were randomly assigned to receive two injections of mRNA-1273 (50 μg each) or placebo and were followed for a median of 82 days (interquartile range, 14 to 94) after the first injection. This dose level was associated with mainly low-grade, transient adverse events, most commonly injection-site pain, headache, and fatigue. No vaccine-related serious adverse events, multisystem inflammatory syndrome in children, myocarditis, or pericarditis were reported as of the data-cutoff date. One month after the second injection (day 57), the neutralizing antibody titer in children who received mRNA-1273 at a 50-μg level was 1610 (95% confidence interval [CI], 1457 to 1780), as compared with 1300 (95% CI, 1171 to 1443) at the 100-μg level in young adults, with serologic responses in at least 99.0% of the participants in both age groups, findings that met the prespecified noninferiority success criterion. Estimated vaccine efficacy was 88.0% (95% CI, 70.0 to 95.8) against Covid-19 occurring 14 days or more after the first injection, at a time when B.1.617.2 (delta) was the dominant circulating variant. CONCLUSIONS Two 50-μg doses of the mRNA-1273 vaccine were found to be safe and effective in inducing immune responses and preventing Covid-19 in children 6 to 11 years of age; these responses were noninferior to those in young adults. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; KidCOVE ClinicalTrials.gov number, NCT04796896. opens in new tab.) Published in NEJM (May 11, 2022): https://doi.org/10.1056/NEJMoa2203315

|
Scooped by
Juan Lama
|
A Fourth Dose of mRNA Vaccine in Health Care Workers Health care workers in Israel were given a fourth dose of BNT162b2 or mRNA-1273 vaccine during a period of omicron-variant predominance. Level. In this open-label, nonrandomized clinical study, we assessed the immunogenicity and safety of a fourth dose of either BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) administered 4 months after the third dose in a series of three BNT162b2 doses (ClinicalTrials.gov numbers, NCT05231005. opens in new tab and NCT05230953. opens in new tab; the protocol is available with the full text of this letter at NEJM.org). Of the 1050 eligible health care workers enrolled in the Sheba HCW COVID-19 Cohort, 154 received the fourth dose of BNT162b2 and, 1 week later, 120 received mRNA-1273. For each participant, two age-matched controls were selected from the remaining eligible participants (Fig. S1 in the Supplementary Appendix, available at NEJM.org). After the fourth dose, both messenger RNA (mRNA) vaccines induced IgG antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain and increased neutralizing antibody titers (Fig. S3); each measure was increased by a factor of 9 to 10, to titers that were slightly higher than those achieved after the third dose, with no significant difference between the two vaccines. Concurrently, antibody levels in the control group continued to wane (Table S5). Both vaccines induced an increase in live neutralization of the B.1.1.529 (omicron) variant and other viral strains by a factor of approximately 10 , similar to the response after the third dose. We found that the fourth dose did not lead to substantial adverse events despite triggering mild systemic and local symptoms in the majority of recipients (Fig. S2 and Table S4A and S4B). Because of the extremely high infection incidence and meticulous active surveillance with weekly SARS-CoV-2 polymerase-chain-reaction testing, we were also able to assess vaccine efficacy with a Poisson regression model (see the Supplementary Appendix). Overall, 25.0% of the participants in the control group were infected with the omicron variant, as compared with 18.3% of the participants in the BNT162b2 group and 20.7% of those in the mRNA-1273 group. Vaccine efficacy against any SARS-CoV-2 infection was 30% (95% confidence interval [CI], −9 to 55) for BNT162b2 and 11% (95% CI, −43 to 44) for mRNA-1273. Most infected health care workers reported negligible symptoms, both in the control group and the intervention groups. However, most of the infected participants were potentially infectious, with relatively high viral loads (nucleocapsid gene cycle threshold, ≤25) (Table S6). Vaccine efficacy was estimated to be higher for the prevention of symptomatic disease (43% for BNT162b2 and 31% for mRNA-1273) (Fig. S4). Limitations of the study include its nonrandomized design and the 1-week difference between enrollment in the two intervention groups, generating potential biases. To overcome this, we assessed each intervention group separately and used a Poisson model accounting for calendar time. In addition, despite similar requests for weekly SARS-CoV-2 testing, adherence was slightly lower in the control group. We did not sequence the infecting virus and cannot be absolutely certain that all cases were caused by the omicron variant; however, during the study period, omicron accounted for 100% of the isolates that were typed. Finally, our cohort was too small to allow for accurate determination of vaccine efficacy. However, within the wide confidence intervals of our estimates, vaccine efficacy against symptomatic disease was 65% at most. Our data provide evidence that a fourth dose of mRNA vaccine is immunogenic, safe, and somewhat efficacious (primarily against symptomatic disease). A comparison of the initial response to the fourth dose with the peak response to a third dose did not show substantial differences in humoral response or in levels of omicron-specific neutralizing antibodies. Along with previous data showing the superiority of a third dose to a second dose, our results suggest that maximal immunogenicity of mRNA vaccines is achieved after three doses and that antibody levels can be restored by a fourth dose. Furthermore, we observed low vaccine efficacy against infections in health care workers, as well as relatively high viral loads suggesting that those who were infected were infectious. Thus, a fourth vaccination of healthy young health care workers may have only marginal benefits. Older and vulnerable populations were not assessed. Published in NEJM (March 16, 2022): https://doi.org/10.1056/NEJMc2202542

|
Scooped by
Juan Lama
|
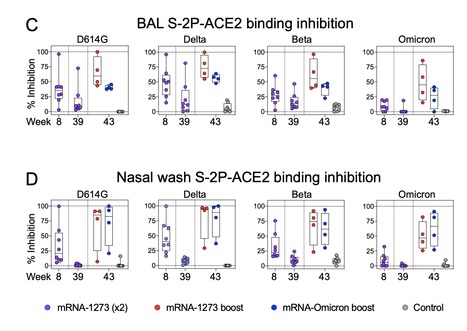
SARS-CoV-2 Omicron is highly transmissible and has substantial resistance to antibody neutralization following immunization with ancestral spike-matched vaccines. It is unclear whether boosting with Omicron-specific vaccines would enhance immunity and protection. Here, nonhuman primates that received mRNA-1273 at weeks 0 and 4 were boosted at week 41 with mRNA-1273 or mRNA-Omicron. Neutralizing antibody titers against D614G were 4760 and 270 reciprocal ID50 at week 6 (peak) and week 41 (pre-boost), respectively, and 320 and 110 for Omicron. Two weeks after boost, titers against D614G and Omicron increased to 5360 and 2980, respectively, for mRNA-1273 and 2670 and 1930 for mRNA-Omicron. Following either boost, 70-80% of spike-specific B cells were cross-reactive against both WA1 and Omicron. Significant and equivalent control of virus replication in lower airways was observed following either boost. Therefore, an Omicron boost may not provide greater immunity or protection compared to a boost with the current mRNA-1273 vaccine. Preprint available in bioRxix (Feb.04, 2022): https://doi.org/10.1101/2022.02.03.479037
|

|
Scooped by
Juan Lama
|
Moderna's vaccine and new shots from Pfizer and Novavax are slated to roll out within weeks, pending potential approvals from the Food and Drug Administration. Moderna’ new Covid vaccine generated a robust immune response against the now-dominant Eris variant and another rapidly spreading strain of the virus in an early clinical trial, the biotech company said Thursday. The updated shot is designed to target omicron subvariant XBB.1.5, but the results suggest that the jab may still be effective against newer variants of the virus that are gaining ground nationwide. That includes Eris and another variant nicknamed Fornax, both of which are also descendants of the omicron virus variant. Moderna’s vaccine and new shots from Pfizer and Novavax are slated to roll out within weeks, pending potential approvals from the U.S. Food and Drug Administration. Meanwhile, Covid-related hospitalizations fueled by Eris and other variants continue to accelerate but remain below the summer peak that strained hospitals this time last year. Eris, also known as EG.5, accounted for 17.3% of all cases as of earlier this month, according to the Centers for Disease Control and Prevention. The World Health Organization designated Eris a “variant of interest,” meaning it will be monitored for mutations that could make it more severe. Fornax, or FL 1.5.1, is also beginning to surge in parts of t he U.S. It accounted for 8.6% of all cases nationwide as of earlier this month, the CDC said. A Pfizer spokesperson on Thursday said the company’s own updated Covid shot effectively neutralized XBB.1.5 and Eris, among other variants, in a recent trial on mice.

|
Scooped by
Juan Lama
|
Back in January, Moderna set a planned mpox vaccine on the back burner as the immediate outbreak waned, but now the shot has slid into the priority lane, with entry to the clinic likely a “month or | Back in January, Moderna set a planned mpox vaccine on the back burner as the immediate outbreak waned, but now the shot has slid into the priority lane, with entry to the clinic likely a “month or so” away, according to a top vaccine developer and strategist at the company. Moderna is set to launch a phase 1/2 trial of the shot this summer, Hamilton Bennett, senior director of vaccine access and partnerships at Moderna, said in an interview at the the BIO International Convention. The company had previously teased launching human trials sometime this year. Bennett said the goal is to build a preclinical data package that shows efficacy across orthopoxviruses, which include mpox and smallpox, and then generate "robust" phase 1/2 data to inform dose selection and eventually licensure. She added that the company has met with the U.S. mpox response team a couple of times to say, “this is where we are, these are the barriers that we're seeing.” “They've been incredibly helpful in helping us get access to samples and people and information,” she said. Moderna CEO Stéphane Bancel told Fierce Biotech in January that developing a mpox vaccine was not a high priority as development pressed ahead on more than a dozen unique assets beyond the COVID vaccine that helped make the company a household name. Bennett clarified that he was speaking relative to Moderna’s urgency to develop a COVID-19 vaccine during the pandemic. “I think when Stéphane says it's not a priority, it is not a COVID-like priority,” she said. The upcoming trials are just the latest example of the swiftness of Moderna's mRNA platform. The company announced in May 2022 that preclinical work would begin on an mpox vaccine. But no update had been made on the progress prior to Bancel's comments at the beginning of the year. For Bennett, the development of the shot means more than just the potential for a new product; it’s an opportunity to flex her muscles leading the company’s public health portfolio, a position she’s pivoted to since helping lead development and dissemination of the COVID shot. That new role broadly puts her in charge of coordinating with developing nations to help scale up vaccine-related economic infrastructure and ensuring that Moderna is accountable and its products are available to those nations. Bennett said that mpox is a “perfect example” of some of the barriers the company will face as it develops these products, in part because, as of now, funding for an mpox vaccine is really for a smallpox vaccine. At a grander level, mpox represents a virus that only gained national attention when people outside of Africa began to be infected. Bennett doesn’t want Moderna to be caught on its back foot when addressing diseases of significance, even if they aren’t affecting high-income countries. “Our portfolio in global health is designed to allow those associations to happen because they're not something where we can pick up the phone when the clock starts,” she said. “We need to build those relationships now.”

|
Scooped by
Juan Lama
|
On September 1, 2022, the Moderna and Pfizer–BioNTech bivalent vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) containing equal amounts of spike messenger RNA from the ancestral and omicron BA.4–BA.5 subvariants replaced their monovalent counterparts as booster doses for persons who are 12 years of age or older in the United States. We previously reported surveillance data from North Carolina on the effectiveness of these two bivalent boosters against coronavirus disease 2019 (Covid-19) during the first 3 months after deployment (September 1 to December 8, 2022); the BA.4–BA.5 subvariants were predominant during the first 2.5 months of this period.1 Here, we present two additional months of data that were obtained during a period when the omicron BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants had become predominant to show the durability of protection conferred by these two bivalent boosters against a wider range of clinical outcomes than were included in our previous report. The data sources and study design have been described previously,1-3 and updated information is provided in the Methods section of the Supplementary Appendix, available with the full text of this letter at NEJM.org. The current study used data regarding booster doses and clinical outcomes from September 1, 2022, to February 10, 2023, for all North Carolina residents who were 12 years of age or older. During this period, a total of 6,306,311 residents were eligible to receive bivalent boosters; of these residents, 1,279,802 received the injections. A total of 19,462 of the 154,581 SARS-CoV-2 infections, 253 of the 2208 Covid-19–related hospitalizations, and 79 of the 867 Covid-19–related deaths occurred after receipt of the bivalent booster (Table S1 in the Supplementary Appendix). We considered four outcome measures: infection, severe infection resulting in hospitalization, severe infection resulting in hospitalization or death, and severe infection resulting in death. We fit the Cox regression model with a time-varying hazard ratio for severe infection and fit the proportional-rates model with a time-varying rate ratio for recurrent infection for each additional booster dose that was received (i.e., first booster vs. primary vaccination, second booster vs. first booster, or third booster vs. second booster); all measures were adjusted for the baseline characteristics shown in Table S1. We estimated the booster effectiveness on a particular day as 1 minus the hazard ratio or rate ratio on that day multiplied by 100%. Effectiveness against severe infection resulting in hospitalization was slightly lower, and effectiveness against infection was much lower. The effectiveness against severe infection resulting in death was the highest despite uncertainty because of the small number of events. We also analyzed the data separately for participants who received bivalent boosters before November 1, 2022 (when the BA.4–BA.5 subvariants were predominant) and after November 1, 2022 (when the BQ.1–BQ.1.1 subvariants were more prevalent and then were gradually replaced by the XBB–XBB.1.5 subvariants). The results are shown in the right column of Figure 1 and in Tables S3 and S4. The effectiveness was broadly similar between the two booster cohorts. Finally, we performed subgroup analyses according to the participant’s age and previous infection status and according to the manufacturers of the bivalent vaccine and the previous vaccine. Effectiveness against infection was higher for the Moderna bivalent vaccine than for the Pfizer–BioNTech bivalent vaccine and higher among previously infected participants than among those with no previous infection (Fig. S1). The two types of bivalent boosters were associated with an additional reduction in the incidence of omicron infection among participants who had previously been vaccinated or boosted. Although the two bivalent vaccines were designed to target the BA.4–BA.5 subvariants, they were also associated with a lower risk of infection or severe infection with the BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants. The effectiveness was higher against hospitalization and death than against infection and waned gradually from its peak over time. Published in NEJM (April 12, 2023): https://doi.org/10.1056/NEJMc2302462

|
Scooped by
Juan Lama
|
US biotech company Moderna on Tuesday announced positive interim trial results for its vaccine against respiratory syncytial virus (RSV) in adults over the age of 60. There are no jabs currently available for the virus, which is a top cause of lower respiratory diseases, commonly leading to bronchiolitis in children and pneumonia in the elderly. However numerous vaccines and treatments are under development, most notably by Moderna's rival COVID-19 vaccine maker Pfizer. Moderna's vaccine was found to be nearly 84 percent effective against RSV-linked diseases in Phase III trials—the final stage of human testing—the firm said in a statement. The trial of the vaccine, which uses the new mRNA technology from Moderna's COVID jab, involves some 37,000 adults over 60 in 22 countries including the United States. The company released the findings of an interim analysis, which has not been peer-reviewed. In the placebo group, there were 55 cases of RSV-linked lower respiratory tract disease with at least two symptoms, compared to nine in the group that received the vaccine, it said. The vaccine had no serious side effects, the company added. Moderna plans to apply for regulatory approval for the vaccine in the US, Europe and other regions in the coming months. This could make it available for the RSV season in the Northern Hemisphere's winter this year, Moderna's chief medical officer Paul Burton told AFP. The company is also testing the vaccine for use in children, but those trials are still at the first phase. 'Good news' In high-income countries, RSV caused 5.2 million cases of severe disease among adults over 60 in 2019, Moderna said. Up to 30,000 elderly patients die every year in G7 countries due to the virus, Burton added. He said that the number of doses required for Moderna's vaccine was yet to determined. The positive results come after Pfizer announced in December that its own RSV vaccine for over-60s was granted priority review status by the US Food and Drug Administration. The previous month, Pfizer said another of its RSV vaccines, which is given to pregnant mothers, was effective at protecting newborns. Also in November, the European Union approved a preventative treatment which works similarly to a vaccine made by AstraZeneca and Sanofi which has been shown to prevent severe illness from RSV in infants. When asked about other RSV vaccines being developed by Moderna's rivals, Burton said "it's good news". "The public has gone seven decades with nothing" to fight the virus, and soon could have multiple options, he said. Moderna is also looking at whether the RSV jab can be combined with COVID and even influenza vaccines, after soaring cases of all three in recent months was dubbed a "tripledemic". Moderna's Press Release: https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx

|
Scooped by
Juan Lama
|
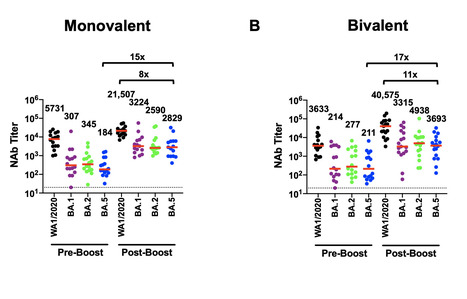
Moderna said its new booster appears to be more effective against Omicron variants than its original vaccine, and that it also seems effective against a new worrisome variant, BQ1.1. New data indicate Moderna’s Covid bivalent booster may be more effective against currently circulating Omicron variants of the virus than its original vaccine, the company said Monday. Like data released by Pfizer and BioNTech regarding their Covid vaccine, the new data involves lab measurements of antibodies and their ability to neutralize the SARS-CoV-2 virus, not data on how well the vaccines prevent cases of symptomatic illness or severe disease. Moderna said in a press release that giving its current booster led to an increase in the number of antibodies that neutralize the most common Omicron variants, BA.4 and BA.5, by 15-fold. The data have not been published in a peer-reviewed journal or released in a preprint. That meant that the geometric mean titers of BA.4/BA.5 antibodies were 5.11-fold higher for those who received the new booster compared to those who received a booster of the original Moderna Covid vaccine if those people had been previously infected with Covid. For those without previous Covid infection, those numbers were 6.29 times higher than with the original vaccine. One caveat is that with previous studies of experimental Moderna boosters that targeted new strains of the virus, it was possible to compare volunteers who received the original vaccine and the modified one at the same time. But the new booster, which targets both the original strain of the SARS-CoV-2 virus and the Omicron sub-variants BA.4/BA.5, was authorized by the Food and Drug Administration before such a study was conducted. Jacqueline Miller, a Moderna senior vice president, said that the company did not think it was ethical to enroll a new cohort on the original Moderna booster when the recommendations from the FDA and the Centers for Disease Control and Prevention were that people should get a booster that is more targeted at the BA.4/BA.5 strains. So far, results have been mixed as to whether the BA.4/BA.5 boosters are more effective against the new strains than the original shots. Studies conducted in the laboratories of the vaccine researcher Daniel Barouch, of Beth Israel Deaconess Medical Center, and the virologist David Ho, of Columbia University, have indicated that the new shots may not be more effective than the original one. But studies conducted at the University of Texas and at Emory University have shown that the new shots may yield better antibody protection. Miller emphasized that Moderna has used consistent methods for its antibody studies, including for the earlier studies that showed the original vaccine was effective, and that those studies have been greenlit by the FDA. Eric Topol, director and founder of the Scripps Research Translational Institute, said that he viewed the new results as positive and largely confirmatory of the Emory work. He also praised the study for its relatively large size, involving 511 previously vaccinated volunteers. “This study is encouraging and suggests that the bivalent boosters provide an added benefit compared to the prior mRNA-1273 booster,” said Mehul Suthar, lead author of the Emory analysis, in an email. “We will need to stay up to date with this bivalent booster to protect against yet another Omicron wave this fall/winter.” Moderna also said that the new booster appeared to be effective against a new worrisome variant, BQ1.1, in a preliminary study using samples from 40 of the study’s volunteers. “What people have been looking for is an understanding of, ‘if I got this booster what does my protection look like against BQ1.1 or some other variant,’” Miller said. “Ultimately the effectiveness data are the really important data.” She said that Moderna expects data from an ongoing observational study to help answer that question in the first quarter of next year.

|
Scooped by
Juan Lama
|
Waning immunity following mRNA vaccination and the emergence of SARS-CoV-2 variants has led to reduced mRNA vaccine efficacy against both symptomatic infection and severe disease. Bivalent mRNA boosters expressing the Omicron BA.5 and ancestral WA1/2020 Spike proteins have been developed and approved, because BA.5 is currently the dominant SARS-CoV-2 variant and substantially evades neutralizing antibodies (NAbs). Our data show that BA.5 NAb titers were comparable following monovalent and bivalent mRNA boosters. Preprint available in bioRxiv: https://doi.org/10.1101/2022.10.24.513619

|
Scooped by
Juan Lama
|
BACKGROUND The safety and immunogenicity of the bivalent omicron-containing mRNA-1273.214 booster vaccine are not known. METHODS In this ongoing, phase 2–3 study, we compared the 50-μg bivalent vaccine mRNA-1273.214 (25 μg each of ancestral Wuhan-Hu-1 and omicron B.1.1.529 [BA.1] spike messenger RNAs) with the previously authorized 50-μg mRNA-1273 booster. We administered mRNA-1273.214 or mRNA-1273 as a second booster in adults who had previously received a two-dose (100-μg) primary series and first booster (50-μg) dose of mRNA-1273 (≥3 months earlier). The primary objectives were to assess the safety, reactogenicity, and immunogenicity of mRNA-1273.214 at 28 days after the booster dose. RESULTS Interim results are presented. Sequential groups of participants received 50 μg of mRNA-1273.214 (437 participants) or mRNA-1273 (377 participants) as a second booster dose. The median time between the first and second boosters was similar for mRNA-1273.214 (136 days) and mRNA-1273 (134 days). In participants with no previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the geometric mean titers of neutralizing antibodies against the omicron BA.1 variant were 2372.4 (95% confidence interval [CI], 2070.6 to 2718.2) after receipt of the mRNA-1273.214 booster and 1473.5 (95% CI, 1270.8 to 1708.4) after receipt of the mRNA-1273 booster. In addition, 50-μg mRNA-1273.214 and 50-μg mRNA-1273 elicited geometric mean titers of 727.4 (95% CI, 632.8 to 836.1) and 492.1 (95% CI, 431.1 to 561.9), respectively, against omicron BA.4 and BA.5 (BA.4/5), and the mRNA-1273.214 booster also elicited higher binding antibody responses against multiple other variants (alpha, beta, gamma, and delta) than the mRNA-1273 booster. Safety and reactogenicity were similar with the two booster vaccines. Vaccine effectiveness was not assessed in this study; in an exploratory analysis, SARS-CoV-2 infection occurred in 11 participants after the mRNA-1273.214 booster and in 9 participants after the mRNA-1273 booster. CONCLUSIONS The bivalent omicron-containing vaccine mRNA-1273.214 elicited neutralizing antibody responses against omicron that were superior to those with mRNA-1273, without evident safety concerns. (Funded by Moderna; ClinicalTrials.gov number, NCT04927065. opens in new tab.) Published in NEJM (Sept.16, 2022): https://doi.org/10.1056/NEJMoa2208343

|
Scooped by
Juan Lama
|
The bivalent vaccine will now form part of the booster campaign to be rolled out this autumn. Ministers say the vaccine will now form part of the autumn booster campaign. Moderna thinks 13 million doses of its new vaccine will be available this year, but 26 million people are eligible for some form of booster. Health officials say people should take whichever booster they are offered as all jabs provide protection. The original vaccines used in the pandemic were designed to train the body to fight the first form of the virus which emerged in Wuhan, in China, at the end of 2019. The Covid virus has since mutated substantially, with a stream of new variants emerging that can dodge some of our immune defences. They have caused large surges in cases around the world. The original vaccines still provide strong protection against becoming severely ill or dying, but companies are tweaking them to match the virus as it evolves. Cases of coronavirus are currently falling in the UK. In mid-to-late July, around 2.5 million people tested positive for coronavirus. 'Sharpened tool' Moderna's latest vaccine - called Spikevax - targets both the original strain and the first Omicron variant (BA.1), which emerged last winter. It is known as a bivalent vaccine as it takes aim at two forms of Covid. The UK's Medicines and Healthcare Products Regulatory Agency has considered the evidence and given the vaccine approval for use in adults. Dr June Raine, the regulator's chief executive, said: "What this bivalent vaccine gives us is a sharpened tool in our armoury to help protect us against this disease as the virus continues to evolve." Experiments on 437 people showed the updated vaccine was safe and gave better immune protection against newer variants. Levels of antibodies that were able to stick to and disable Omicron (BA.1) were 1.7 times higher in people given the new vaccine. Tests against more recent Omicron variants (BA.4 and BA.5), which are causing the UK's current wave, also showed higher levels of protection with the updated vaccine. However, it is far from clear what that means in terms of preventing someone from becoming seriously ill. Additionally, it is uncertain what variants we will be facing in the coming months and exactly how well the updated vaccine will perform against them. Health ministers have officially given the go-ahead for the bivalent vaccines. In England, Health Secretary Steve Barclay said it was "very good news for the UK population" and those eligible "will have the comfort of knowing that their immunity has been topped up". People will be contacted from early September, he said. Wales' health minister Eluned Morgan said vaccines "have saved countless lives" and urged everyone who was eligible to come forward. The Joint Committee on Vaccination and Immunisation (JCVI), which advises governments in England, Wales, Northern Ireland and Scotland, has confirmed the following groups should be offered some form of booster in the autumn: - health and social care staff
- everyone aged 50 and over
- carers who are over the age of 16
- people over five whose health puts them at greater risk, this includes pregnant women
- people over five who share a house with somebody with a weakened immune system
Stéphane Bancel, the chief executive officer of Moderna, said he was "delighted" the vaccine had been approved. "This represents the first authorisation of an Omicron-containing bivalent vaccine; this bivalent vaccine has an important role to play in protecting people in the UK from Covid-19 as we enter the winter months," he said. Prof Wei Shen Lim, from the JCVI, said: "It is important that everyone who is eligible takes up a booster this autumn, whichever vaccine is on offer." Who gets a winter booster Originally those aged 50-65 were not going to be jabbed. However, the immunisation campaign has been expanded because of the rapid spread of variants, uncertainty about how the virus will mutate and the expectation that we will are likely to be more social - and therefore give the virus a helping hand this winter - including at Christmas. However, most people under 50 will not be boosted in the coming months. The focus remains on preventing those most at risk from becoming seriously ill, rather than stopping the young passing the virus on to older relatives. Moderna is not the only company updating its vaccines. Pfizer has also been developing vaccines that can target Omicron. The Oxford-AstraZeneca vaccine, however, is not being updated.

|
Scooped by
Juan Lama
|
As Pfizer and Moderna's COVID-19 vaccine applications in young children move through the FDA's regulatory process, parents may soon be able to line their children up for shots. | As Pfizer and Moderna's COVID-19 vaccine applications in young children move through the FDA's regulatory process, parents may soon be able to line their children up for shots. At an advisory committee this week, independent experts will vote on whether to endorse Moderna’s vaccine in a wide range of children and adolescents and Pfizer’s in children 6 months through 4 years old. Ahead of the meeting, the FDA staffers posted their own findings, concluding that the vaccines are generally safe and effective in the respective age groups. Data on Pfizer's program showed that the three-dose series in children 6 months through 23 months of age was 75.6% effective, the reviewers said. Efficacy for the 2- to 4-years-old age group was 82.4%. The main adverse reactions in the 6-23 months group was tenderness at the injection site, irritability, drowsiness, decreased appetite and fever. Rates of adverse reactions for those recipients were lower than those in the 5- to 11-years-old age group, the FDA staff said. In the 2- to 4-year-old age group, adverse reactions included pain at the injection site, fatigue, headache and chills. Again, the rates were lower than those in the 5- to 11-years old group. In response to the FDA’s request for additional data on the vaccine's effectiveness against the delta and omicron variants, an analysis found the vaccine elicits a similar level of antibodies against delta and the reference strain, but noticeably lower levels against omicron. As for Moderna's program, the vaccine is not yet authorized for adolescents, so Moderna is seeking a nod in children 6 months to 17 years old in two separate applications—one for children 6 months to 5 years and another in children 6 years to 17-years-old. Trials showed that vaccine efficacy for recipients 12- to 17-years-old was 93.3%, which is largely consistent with the efficacy in the adult study. The 6- to 11-years-old group saw an efficacy rate of 76.8%. The Moderna studies were conducted during the omicron surge and efficacy for this variant appeared consistent with efficacy observed among adults during the surge, the FDA staffers said. Meanwhile, efficacy in the 2- to 5-years-old group was lower at 36.8% using the CDC case definition. Finally in the 6 months through 23 months group, efficacy under the CDC definition was 50.6%. The most common reaction after receiving a dose was injection site pain, followed by headaches and fatigue. Other adverse reactions matched the placebo dose. Irritability or crying was another frequently reported and persistent reaction. COVID-19 doesn’t show signs of stopping with over 533 million cases and 6.3 million deaths worldwide, according to the CDC. If the FDA authorizes the children’s vaccines, shots could start going in arms by June 21, the Biden administration has said.

|
Scooped by
Juan Lama
|
The omicron (B.1.1.529) variant, first detected in the UK on Nov 27, 2021, rapidly became the dominant strain, due in part to reduced vaccine effectiveness. An increase in sequenced cases of the omicron sub-lineage BA.2 was observed in the week beginning on Jan 3, 2022. BA.2 has a growth advantage over BA.1, and has become the dominant strain in the UK at the time of writing. Neutralisation assays using monoclonal antibodies have suggested a small antigenic difference between BA.1 and BA.2, although sera from individuals with booster vaccinations neutralise both variants similarly. The UK COVID-19 vaccination programme has been in place since Dec 8, 2020, with primary courses of two doses of either BNT162b2 (Comirnaty, Pfizer–BioNTech), ChAdOx1-S (Vaxzevria, Oxford/AstraZeneca), or mRNA-1273 (Spikevax, Moderna). Booster vaccination with either BNT162b2 or a half dose (50 μg) of mRNA-1273 was introduced on Sept 14, 2021, to adults older than 50 years and those in risk groups, and on Nov 29, 2021, to all adults. In this Comment, we estimate vaccine effectiveness against symptomatic disease and hospitalisation with BA.1 and BA.2 after one or two doses of BNT162b2, ChAdOx1-S, or mRNA-1273, and after booster doses of BNT162b2 or mRNA-1273 during a period of co-circulation. We used a test-negative case-control study design. Our analysis included all vaccines used in the UK. Vaccination status was included as an independent variable and effectiveness defined as 1 minus the odds of vaccination in cases, divided by the odds of vaccination in controls..... Published in The Lancet Infectious Diseases (May 24, 2022):

|
Scooped by
Juan Lama
|
Comparison of the immune response to four prominent COVID-19 vaccines is among the most thorough so far, authors say. A rare head-to-head comparison shows that the COVID-19 vaccines made by Pfizer and Moderna outperform those from Johnson & Johnson and Novavax1. The data also provide a finely detailed picture of the immune protection that each vaccine offers — information that could be useful for designing future vaccines. The research was posted on the preprint server bioRxiv on 21 March. It has not yet been peer reviewed. The study assessed the 4 vaccines using 14 metrics, including levels of several types of immune cell such as T cells and B cells, as well as immune molecules called neutralizing antibodies. Such investigations are sorely needed to sort through the flood of COVID-19 vaccines in the research pipeline and on the market, researchers say. “It’s a really nice analysis by premier immunologists that builds upon what has been previously shown,” says Robert Seder, an immunologist at the US National Institute of Allergy and Infectious Diseases in Bethesda, Maryland. Previous comparisons of COVID-19 vaccines have often brought together data from different studies, which might have been conducted with slightly varying laboratory techniques. For the latest study, by contrast, researchers applied the same techniques across all the vaccines they investigated. “When you try to compare [vaccine data] between different papers, which is what many of us have been doing for over a year now, you get apples to oranges comparisons, and you can be way off,” says Shane Crotty, an immunologist at La Jolla Institute for Immunology in California and a co-author of the preprint. The four vaccines that Crotty and his co-authors examined fall into three classes. The jabs made by Moderna in Cambridge, Massachusetts, and by Pfizer in New York City and BioNTech in Mainz, Germany, are both based on messenger RNA. Johnson & Johnson (J&J) of New Brunswick, New Jersey, has produced a ‘viral vector’ vaccine that uses a harmless virus to deliver SARS-CoV-2 genetic material into host cells. The vaccine made by Novavax in Gaithersburg, Maryland, contains pieces of the SARS-CoV-2 spike protein. Strengths and weaknesses Antibody levels induced by two doses of Pfizer’s or Moderna’s mRNA vaccine tended to wane substantially over six months. By contrast, antibody levels from J&J’s one-shot vaccine were stable or even increased over time. But antibody levels measured six months after vaccination with the J&J jab were still lower than those observed six months after vaccination with an mRNA vaccine. Novavax’s two-shot regimen induced antibody responses on a par with those to the mRNA vaccines. However, after the Novavax jab, levels of CD8+ T cells, which destroy infected cells, were low to undetectable, whereas the other three vaccines performed well in this metric. These results generally support the findings of previous studies. But the latest research offers a more extensive analysis of the immune system’s response than do earlier studies, and uses an apples-to-apples approach. No losers “This is not meant to proclaim winners and losers,” says study co-author Alessandro Sette, an immunologist at La Jolla. Instead, the study is meant to “provide a comprehensive evaluation of the different variables”, he says. Novavax has received authorization for its vaccine in 38 countries. The vaccines made by Moderna, Pfizer and J&J have all received wide authorization globally. One caveat is that the study looked at the effects of the Novavax jab in only 12 people. It examined the other three vaccines in 30 volunteers each. Seder notes that the analysis considers the effects of only a two-dose regimen of the mRNA vaccines. It does not consider the protection provided by boosters, because the authors began the work in late 2020, before a third shot was recommended by health authorities. Crotty, Sette and their colleagues are now conducting a similar head-to-head study that includes mRNA boosters. Cited research available at bioRxiv (March 21, 2022): https://doi.org/10.1101/2022.03.18.484953

|
Scooped by
Juan Lama
|
With as much turmoil and negativity as the COVID-19 pandemic has supplied, there may be a silver lining when it comes to a new vaccine study for those suffering with HIV. The first study participant has been enrolled in a new Phase 1 clinical trial using the messenger ribonucleic acid, or mRNA, vaccine technology developed by Moderna to evaluate the safety and immune responses of three different experimental vaccines against HIV. This randomized, open-label trial represents one of the first clinical studies of the use of mRNA vaccine technology against HIV. The University of Alabama at Birmingham enrolled the trial’s second participant and has enrolled several others since. Paul Goepfert, M.D., director of the Alabama Vaccine Research Clinic in the Marnix E. Heersink School of Medicine, says the emergence of mRNA vaccines to combat COVID-19 certainly helped move this mRNA HIV vaccine trial along. “Despite tremendous advancements in treatment and prevention of HIV, we still do not have an effective vaccine and thousands of Americans continue to be infected every year,” Goepfert said. “mRNA technology enables a much more rapid development of vaccines that was not previously possible. This new technology will certainly decrease the time it will take to develop an effective HIV vaccine.” The study, HVTN 302, will enroll up to 108 HIV-negative adults. The primary study hypothesis is that the mRNA vaccines will be safe and well-tolerated among HIV-negative people and will elicit neutralizing antibodies. The experimental vaccines carry mRNA, a piece of genetic code, delivered with instructions for making proteins in the same way that the mRNA vaccines against COVID-19 instruct the body’s cells to make the SARS-CoV-2 spike protein. These instructions show human muscle cells how to make small portions of proteins that resemble parts of HIV but are not the actual virus. People cannot contract HIV from the vaccines. Once immune cells have used the instructions, the mRNA is quickly broken down, and does not stay in the body. The investigational vaccines are not expected to provide protection from HIV infection, yet the knowledge gained from this study will aid in the future development of an HIV vaccine regimen. Researchers hope to learn whether the immune system will respond to the experimental vaccines by making antibodies and T cells that could fight HIV if a person is ever exposed to the virus in the future. The trial will also clarify whether the immune response to an mRNA vaccine is equal to or better than the response to a protein-based vaccine, while helping define the potentials of using mRNA to increase the pace of developing an HIV vaccine. UAB is one of 10 sites nationwide, and the sole site in the South, to enroll participants. At UAB, Goepfert holds the Edward W. Hook, III, M.D., Endowed Professorship in Infectious Diseases.
|



 Your new post is loading...
Your new post is loading...