 Your new post is loading...

|
Scooped by
Juan Lama
|
For cardiologist Eric Topol, this week’s vaccine news presented a personal dilemma. Topol, who directs the Scripps Research Translational Institute and is a popular commenter on COVID-19 research, had hoped to get an updated COVID-19 vaccine from Novavax, rather than a messenger RNA (mRNA) shot from Pfizer or Moderna. Novavax relies on an older, protein-based approach that has shown long-lasting effects against other pathogens, and Topol wondered whether it might produce more durable protection. On Tuesday, it seemed he might get his chance: a drugstore he visited for an mRNA vaccine ran out of doses, and hours later the U.S. Food and Drug Administration authorized a Novavax shot well-matched to current COVID-19 variants. The green light marks the first time Novavax will be widely available to teens and adults. “It’s hard to know how it compares” to mRNA vaccines, Topol admits; there are no head-to-head studies to rely on. In clinical trials, Novavax appeared less likely than mRNA shots to cause side effects like headache and fatigue. But how does it stack up against mRNA vaccines when it comes to protection against SARS-CoV-2? The question has been vexingly difficult to answer. Some hints are emerging, including the first large study of Novavax in the real world, published this week by a team in Italy. The results are far from definitive, but they suggest “there aren’t massive differences” between the vaccines, says Alberto Mateo Urdiales, an epidemiology and infectious disease researcher at the Italian National Institute of Health, who led the study. Whereas mRNA vaccines carry instructions for making a SARS-CoV-2 protein, Novavax directly delivers a fragment of that viral spike protein with an adjuvant for boosting immune response. Such protein subunit vaccines have yielded durable protection against various pathogens including hepatitis B and shingles, along with some respiratory ailments such as pneumonia. A version of the Novavax vaccine targeting the original SARS-CoV-2 variant was approved as a primary vaccination series and first booster in the United States in 2022; it also became available in Europe that year. Its tried-and-true technology appealed to some people wary of the new mRNA approach. And unlike the more fragile mRNA shots, it lasts for months in the refrigerator. But uptake has been low and the company is banking on more shots in arms this fall. The Italian team tried to pin down how well the shot actually works, analyzing data on more than 20,000 Italians who had received two doses as their primary vaccine series in 2022. After 4 months, the vaccine was 55% effective at staving off symptoms from a SARS-CoV-2 infection and 28% effective at preventing infection altogether, the researchers reported in JAMA Network Open. That’s roughly comparable to how the mRNA vaccines have performed, Mateo Urdiales says. He cautions that the emergence of SARS-CoV-2 variants, repeated boosting, and swelling numbers of infections make it hard to compare numbers across the studies of effectiveness conducted to date. Smaller studies, meanwhile, have tried to address another reason a Novavax booster appeals to people like Topol: the possibility that “mixing and matching” various COVID-19 vaccines might provide better protection than any single vaccine brand. “There was theoretical hope that since these vaccines work in slightly different ways, they would have different strengths in terms of which part of the immune system they activate best,” says Angela Branche, an infectious disease specialist at the University of Rochester. She co-chairs a mix-and-match study called COVAIL that includes another protein subunit vaccine from the company Sanofi, which is not available in the U.S....

|
Scooped by
Juan Lama
|
Comparison of the immune response to four prominent COVID-19 vaccines is among the most thorough so far, authors say. A rare head-to-head comparison shows that the COVID-19 vaccines made by Pfizer and Moderna outperform those from Johnson & Johnson and Novavax1. The data also provide a finely detailed picture of the immune protection that each vaccine offers — information that could be useful for designing future vaccines. The research was posted on the preprint server bioRxiv on 21 March. It has not yet been peer reviewed. The study assessed the 4 vaccines using 14 metrics, including levels of several types of immune cell such as T cells and B cells, as well as immune molecules called neutralizing antibodies. Such investigations are sorely needed to sort through the flood of COVID-19 vaccines in the research pipeline and on the market, researchers say. “It’s a really nice analysis by premier immunologists that builds upon what has been previously shown,” says Robert Seder, an immunologist at the US National Institute of Allergy and Infectious Diseases in Bethesda, Maryland. Previous comparisons of COVID-19 vaccines have often brought together data from different studies, which might have been conducted with slightly varying laboratory techniques. For the latest study, by contrast, researchers applied the same techniques across all the vaccines they investigated. “When you try to compare [vaccine data] between different papers, which is what many of us have been doing for over a year now, you get apples to oranges comparisons, and you can be way off,” says Shane Crotty, an immunologist at La Jolla Institute for Immunology in California and a co-author of the preprint. The four vaccines that Crotty and his co-authors examined fall into three classes. The jabs made by Moderna in Cambridge, Massachusetts, and by Pfizer in New York City and BioNTech in Mainz, Germany, are both based on messenger RNA. Johnson & Johnson (J&J) of New Brunswick, New Jersey, has produced a ‘viral vector’ vaccine that uses a harmless virus to deliver SARS-CoV-2 genetic material into host cells. The vaccine made by Novavax in Gaithersburg, Maryland, contains pieces of the SARS-CoV-2 spike protein. Strengths and weaknesses Antibody levels induced by two doses of Pfizer’s or Moderna’s mRNA vaccine tended to wane substantially over six months. By contrast, antibody levels from J&J’s one-shot vaccine were stable or even increased over time. But antibody levels measured six months after vaccination with the J&J jab were still lower than those observed six months after vaccination with an mRNA vaccine. Novavax’s two-shot regimen induced antibody responses on a par with those to the mRNA vaccines. However, after the Novavax jab, levels of CD8+ T cells, which destroy infected cells, were low to undetectable, whereas the other three vaccines performed well in this metric. These results generally support the findings of previous studies. But the latest research offers a more extensive analysis of the immune system’s response than do earlier studies, and uses an apples-to-apples approach. No losers “This is not meant to proclaim winners and losers,” says study co-author Alessandro Sette, an immunologist at La Jolla. Instead, the study is meant to “provide a comprehensive evaluation of the different variables”, he says. Novavax has received authorization for its vaccine in 38 countries. The vaccines made by Moderna, Pfizer and J&J have all received wide authorization globally. One caveat is that the study looked at the effects of the Novavax jab in only 12 people. It examined the other three vaccines in 30 volunteers each. Seder notes that the analysis considers the effects of only a two-dose regimen of the mRNA vaccines. It does not consider the protection provided by boosters, because the authors began the work in late 2020, before a third shot was recommended by health authorities. Crotty, Sette and their colleagues are now conducting a similar head-to-head study that includes mRNA boosters. Cited research available at bioRxiv (March 21, 2022): https://doi.org/10.1101/2022.03.18.484953

|
Scooped by
Juan Lama
|
Practicality of protein-based vaccine by Novavax may help fill a global need, observers say. The dark horse vaccine company Novavax announced strong results today from a pivotal, 30,000-person trial of its pandemic coronavirus vaccine in the United States and Mexico. The vaccine uses a protein of SARS-CoV-2, a different technology than the COVID-19 vaccines authorized so far, and delivered 90.4% overall efficacy against symptomatic COVID-19 infections, and 100% protection against moderate and severe disease. Against eight viral variants of interest and concern, its efficacy was 93.2%. And the vaccine appeared safe and well-tolerated. “This vaccine looks phenomenal. I am thrilled about these results,” says Monica Gandhi, an infectious disease physician and epidemiologist at the University of California, San Francisco. She notes that the clinical trial was highly diverse, with 44% non-white participants, and that the vaccine’s straightforward storage requirements could speed access to it in remote communities around the globe. The difference of a few percentage points between Novavax’s 90% efficacy and the 95% and 94% efficacy of the Pfizer-BioNTech and Moderna vaccines is explained in part by Novavax’s later trial, which pitted the vaccine against viral variants, says John Moore, an immunologist at Weill Cornell Medicine and a participant in the Novavax trial. The trials of those other companies' vaccines, composed of messenger RNA (mRNA), were completed before such variants were widely circulating. “This is a vaccine whose efficacy is at least on a par with Pfizer and Moderna,” Moore says. “It’s essentially 100% protective against disease.” One expert is less impressed, however. “The data they have [are] nothing spectacular,” says Vijay Samant, CEO of Xiconic Pharmaceuticals and a former chief operating officer of Merck’s vaccine division. “The efficacy overall is couple of notches below the Pfizer and Moderna studies. … It’s a me-too vaccine at best.” Novavax plans to apply to the U.S. Food and Drug Administration and other regulators for an emergency use authorization in the third quarter, once the company completes regulatory requirements aimed at ensuring its product consistently matches the vaccine used in the clinical trials, said President and CEO Stanley Erck. Today’s announcement marks the end of a very long beginning for the once-moribund Gaithersburg, Maryland company, which began to develop a vaccine in January 2020, and that July won $1.6 billion from the U.S. government’s Operation Warp Speed. But the company encountered production problems that delayed the launch of its North American clinical trial until late December 2020. In the trial, two-thirds of participants at 113 sites in the United States and six sites in Mexico initially received two doses of the vaccine separated by 21 days. One-third of participants received a placebo. Between January 25 and April 30, participants experienced 77 cases of COVID-19, 63 of them in the placebo group. All 14 cases in vaccinated participants were mild. These results are similar to those Novavax reported in January from a late-stage trial of more than 15,000 people in the United Kingdom. That trial showed the vaccine efficacy was 89% overall, 86% against the Alpha variant that was first identified in the United Kingdom, and 96% against the original virus strain. But in a separate trial involving 4400 participants in South Africa, where the worrisome Beta variant arose and was circulating widely, the vaccine’s overall efficacy sank to 49%. In the new trial, Novavax sequenced 70% of all the coronaviruses that caused illness. In more than half of the sequenced cases, they found the culprit was the Alpha variant, which accounted for 70% of U.S. infections by late April. But the company did not provide specific efficacy data against Alpha or any other variant. (Only two cases of the Beta variant were reported in the trial.) “It doesn’t really address the lingering questions about how well the vaccine would work against ‘escape’ variants” such as Beta, says Natalie Dean, a biostatistician at the University of Florida. The COVID-19 vaccines so far authorized by major Western regulatory agencies deliver genetic material that directs a recipient’s cells to make spike, a surface protein from SARS-CoV-2, that then trains the immune system to respond to the virus. Novavax’s vaccine instead delivers the spike protein itself, carried on soaplike particles and given extra punch by an immune-boosting substance called an adjuvant. Protein technology has been used for decades in vaccines against diseases including hepatitis B. In clinical trials so far, Novovax’s vaccine has produced somewhat fewer powerful, though transient systemwide side effects such as headache, muscle pain, and fatigue, which many recipients of the Pfizer-BioNTech and Moderna vaccines experienced. For instance, 54% of participants who received Moderna’s vaccine as part of a 30,000-person trial reported at least one such effect; in Novavax’s 15,000-person trial in the United Kingdom, that number was 38%. After a second dose of vaccine in the North American Novavax trial, about 40% of participants who received the active vaccine reported experiencing some degree of headache, muscle pain, or fatigue. But serious reactions were rare. Having relatively mild side effects is a plus, says Mayank Mamtani, a biotechnology analyst who follows Novavax for B. Riley Securities. “This Novavax vaccine is going to be less reactogenic. You don’t have to cancel all your meetings” afterward, he says. He adds that the Novavax jab may find a niche as a booster vaccine in the United States, where he says such boosters may be needed by October or November. “We in this country love choice,” he adds. Under its 2020 contract with Operation Warp Speed, Novavax committed to deliver 110 million doses to the U.S. government, although the country does not need them for initial vaccinations. Novavax expects to reach manufacturing capacity of 100 million doses per month by the end of the third quarter and 150 million doses per month by the fourth quarter of 2021, Erck said. The company has advance-purchase orders for about 200 million vaccines from another five Western governments and last month signed a deal to supply Gavi, the Vaccine Alliance, with 350 million doses for the COVID-19 Vaccines Global Access (COVAX) Facility, a global vaccine-sharing partnership. The Serum Institute of India, which is also making the Novavax vaccine, is slated to deliver another 750 million doses to COVAX, Novavax says. The Serum Institute plans to start delivering the Novavax vaccine by September, providing regulatory agencies have authorized it, officials there told Science. Novavax’s production projections are substantially dialed back from earlier company predictions that it would ship 2 billion doses in 2021. That reflects the company’s struggle to scale up its manufacturing both at its own facilities and those of contractors and partner companies in 11 nations. “Novavax has been particularly challenged with executing on production scale-up efforts and manufacturing,” Mamtani wrote in a note to investment clients last month. Unlike mRNA vaccines, which need to be stored frozen, Novavax’s protein vaccine can be stored in a refrigerator for up to 6 months, and, once removed, remains viable for 24 hours. Novavax has created a new version of its vaccine, adapted to the Beta variant, that could be used as a 1-year booster, the company says. On Friday, it announced positive results for the adapted vaccine in mice and baboons. Novavax says it is also working to customize its vaccine to other SARS-CoV-2 variants. But Samant cautions that Novavax’s manufacturing struggles and the entrenched advantages of companies whose vaccines were approved months ago may make the small company’s climb to market share and profitability a tough one.

|
Scooped by
Juan Lama
|
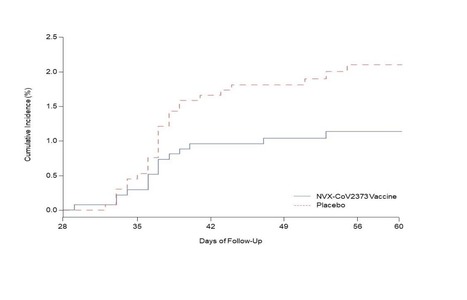
BACKGROUND The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants threatens progress toward control of the coronavirus disease 2019 (Covid-19) pandemic. In a phase 1–2 trial involving healthy adults, the NVX-CoV2373 nanoparticle vaccine had an acceptable safety profile and was associated with strong neutralizing-antibody and antigen-specific polyfunctional CD4+ T-cell responses. Evaluation of vaccine efficacy was needed in a setting of ongoing SARS-CoV-2 transmission. METHODS In this phase 2a–b trial in South Africa, we randomly assigned human immunodeficiency virus (HIV)–negative adults between the ages of 18 and 84 years or medically stable HIV-positive participants between the ages of 18 and 64 years in a 1:1 ratio to receive two doses of either the NVX-CoV2373 vaccine (5 μg of recombinant spike protein with 50 μg of Matrix-M1 adjuvant) or placebo. The primary end points were safety and vaccine efficacy against laboratory-confirmed symptomatic Covid-19 at 7 days or more after the second dose among participants without previous SARS-CoV-2 infection. RESULTS Of 6324 participants who underwent screening, 4387 received at least one injection of vaccine or placebo. Approximately 30% of the participants were seropositive for SARS-CoV-2 at baseline. Among 2684 baseline seronegative participants (94% HIV-negative and 6% HIV-positive), predominantly mild-to-moderate Covid-19 developed in 15 participants in the vaccine group and in 29 in the placebo group (vaccine efficacy, 49.4%; 95% confidence interval [CI], 6.1 to 72.8). Vaccine efficacy among HIV-negative participants was 60.1% (95% CI, 19.9 to 80.1). Of 41 sequenced isolates, 38 (92.7%) were the B.1.351 variant. Post hoc vaccine efficacy against B.1.351 was 51.0% (95% CI, −0.6 to 76.2) among the HIV-negative participants. Preliminary local and systemic reactogenicity events were more common in the vaccine group; serious adverse events were rare in both groups. CONCLUSIONS The NVX-CoV2373 vaccine was efficacious in preventing Covid-19, with higher vaccine efficacy observed among HIV-negative participants. Most infections were caused by the B.1.351 variant. Published in NEJM (May 5, 2021): https://doi.org/10.1056/NEJMoa2103055

|
Scooped by
Juan Lama
|
Background The emergence of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) variants threatens progress toward control of the Covid-19 pandemic. Evaluation of Covid-19 vaccine efficacy against SARS-CoV-2 variants is urgently needed to inform vaccine development and use. Methods In this phase 2a/b, multicenter, randomized, observer-blinded, placebo-controlled trial in South Africa, healthy human immunodeficiency virus (HIV)-negative adults (18 to 84 years) or medically stable people living with HIV (PLWH) (18 to 84 years) were randomized in a 1:1 ratio to receive two doses, administered 21 days apart, of either NVX-CoV2373 nanoparticle vaccine (5 micrograms recombinant spike protein with 50 micrograms Matrix-M1 adjuvant) or placebo. The primary endpoints were safety and vaccine efficacy greater than or equal to 7 days following the second dose against laboratory-confirmed symptomatic Covid-19 in previously SARS-CoV-2 uninfected participants. Results A total of 4387 participants were randomized and dosed at least once, 2199 with NVX CoV2373 and 2188 with placebo. Approximately 30% of participants were seropositive at baseline. Among 2684 baseline seronegative participants (94% HIV negative; 6% PLWH), there were 15 and 29 predominantly mild to moderate Covid-19 cases in NVX CoV2373 and placebo recipients, respectively; vaccine efficacy was 49.4% (95% confidence interval [CI]: 6.1 to 72.8). Efficacy in HIV negative participants was 60.1% (95% CI: 19.9 to 80.1), and did not differ by baseline serostatus. Of the primary endpoint cases with available whole genome sequencing, 38 (92.7%) of 41 were the B.1.351 variant. Post-hoc vaccine efficacy against B.1.351 was 51.0% (95% CI: -0.6 to 76.2) in HIV-negative participants. Among placebo recipients, the incidence of symptomatic Covid-19 was similar in baseline seronegative vs baseline seropositive participants during the first 2 months of follow-up (5.3% vs 5.2%). Preliminary local and systemic reactogenicity were primarily mild to moderate and transient, and higher with NVX CoV2373; serious adverse events were rare in both groups. Conclusions The NVX-CoV2373 vaccine was efficacious in preventing Covid-19, which was predominantly mild to moderate and due to the B.1.351 variant, while evidence of prior infection with the presumptive original SARS CoV-2 did not confer protection against probable B.1.351 disease. (Funded by Novavax, The Bill and Melinda Gates Foundation, and the Coalition for Epidemic Preparedness Innovations; ClinicalTrials.gov number, NCT04533399) Preprint available in medRxiv (March 3, 2021): https://doi.org/10.1101/2021.02.25.21252477

|
Scooped by
Juan Lama
|
NIH- and BARDA-funded trial will enroll up to 30,000 volunteers. The Phase 3 trial of another investigational coronavirus disease 2019 (COVID-19) vaccine has begun enrolling adult volunteers. The randomized, placebo-controlled trial will enroll approximately 30,000 people at approximately 115 sites in the United States and Mexico. It will evaluate the safety and efficacy of NVX-CoV2373, a vaccine candidate developed by Novavax, Inc., of Gaithersburg, Maryland. Novavax is leading the trial as the regulatory sponsor. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response, are funding the trial. “Addressing the unprecedented health crisis of COVID-19 has required extraordinary efforts on the part of government, academia, industry and the community,” said NIAID Director Anthony S. Fauci, M.D. “The launch of this study — the fifth investigational COVID-19 vaccine candidate to be tested in a Phase 3 trial in the United States — demonstrates our resolve to end the pandemic through development of multiple safe and effective vaccines.” The trial is being conducted in collaboration with Operation Warp Speed(link is external) (OWS), a multi-agency collaboration overseen by HHS and the Department of Defense that aims to accelerate development, manufacture and distribution of medical countermeasures for COVID-19. Some of the U.S. trial sites participating are part of the NIAID-supported COVID-19 Prevention Network(link is external) (CoVPN). The CoVPN includes existing NIAID-supported clinical research networks with infectious disease expertise and was designed for rapid and thorough evaluation of vaccine candidates and monoclonal antibodies for preventing COVID-19. Volunteers will be asked to give informed consent prior to their participation in the trial. They will be grouped into two cohorts: individuals 18 through 64 years old and those aged 65 and older, with a goal of enrolling at least 25% of all volunteers who are 65 years old or older. Trial organizers also are emphasizing recruitment of people who are at higher risk of severe COVID-19 disease, including those who are Black (including African Americans), Native American, or of Latino or Hispanic ethnicity, and people who have underlying health conditions such as obesity, chronic kidney disease or diabetes. “We’ve come this far, this fast, but we need to get to the finish line,” said NIH Director Francis S. Collins, M.D., Ph.D. “That will require multiple vaccines using different approaches to ensure everyone is protected safely and effectively from this deadly disease.” After providing a baseline nasopharyngeal and blood sample, participants will be assigned at random to receive an intramuscular injection of either the investigational vaccine or a saline placebo. Randomization will be in a 2:1 ratio with two volunteers receiving the investigational vaccine for each one who receives placebo. Because the trial is blinded, neither investigators nor participants will know who is receiving the candidate vaccine. A second injection will be administered 21 days after the first. Participants will be followed closely for potential vaccine side effects and will be asked to provide blood samples at specified time points after each injection and during the following two years. Scientists will analyze the blood samples to detect and quantify immune responses to SARS-CoV-2, the virus that causes COVID-19. Of note, specialized assays will be used to distinguish between immunity as a result of natural infection and vaccine-induced immunity. The trial’s primary endpoint is to determine whether NVX-CoV2373 can prevent symptomatic COVID-19 disease seven or more days after the second injection relative to placebo. Novavax’s investigational vaccine, NVX-CoV2373, is made from a stabilized form of the coronavirus spike protein using the company’s recombinant protein nanoparticle technology. The purified protein antigens in the vaccine cannot replicate and cannot cause COVID-19. The vaccine also contains a proprietary adjuvant, MatrixM™. Adjuvants are additives that enhance desired immune system responses to vaccine. NVX-CoV2373 is administered in liquid form and can be stored, handled and distributed at above-freezing temperatures (35° to 46°F). A single vaccine dose contains 5 micrograms (mcg) of protein and 50 mcg of adjuvant. In animal tests, NVX-CoV2373 vaccination produced antibodies that blocked the coronavirus spike protein from binding to the cell surface receptors targeted by the virus, preventing viral infection. In results(link is external) of a Phase 1 clinical trial published in the New England Journal of Medicine, NVX-CoV2373 was generally well-tolerated and elicited higher levels of antibodies than those seen in blood samples drawn from people who had recovered from clinically significant COVID-19. NVX-CoV2373 also is being evaluated in a Phase 2b trial in South Africa, now fully enrolled with 4,422 volunteers, and data from a Phase 1/2 continuation trial in the United States and Australia is expected as early as first quarter 2021. Novavax also recently completed enrollment of more than 15,000 volunteers in a Phase 3 trial of the candidate vaccine in the United Kingdom, which is also testing two injections of 5 mcg of protein and 50 mcg of Matrix-M adjuvant administered 21 days apart. An independent Data and Safety Monitoring Board (DSMB) will provide oversight to ensure the safe and ethical conduct of the study. All Phase 3 clinical trials of candidate vaccines supported through OWS are overseen by a common DSMB developed in consultation with the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative.

|
Scooped by
Juan Lama
|
The trial is expected to enroll and test the vaccine in up to 10,000 participants aged between 18 and 84 years over the next four to six weeks. - Novavax Inc on Thursday started a late-stage trial of its experimental Covid-19 vaccine in partnership with the UK government’s Vaccines Taskforce, sending the company’s shares up 6% after the bell.
- The trial is expected to enroll and test the vaccine in up to 10,000 participants aged between 18 and 84 years over the next four to six weeks.
- Data from the trial will support regulatory submissions for license in the UK, EU and other countries, the company said.
Novavax on Thursday started a late-stage trial of its experimental Covid-19 vaccine in partnership with the UK government’s Vaccines Taskforce, sending the company’s shares up 6% after the bell. The trial is expected to enroll and test the vaccine in up to 10,000 participants aged between 18 and 84 years over the next four to six weeks. Data from the trial will support regulatory submissions for license in the UK, EU and other countries, the company said The study has two main goals, the first occurrence of PCR-confirmed symptomatic Covid-19 at least 7 days after the second study vaccination in volunteers who have not been previously infected with SARS-CoV-2. The second main goal is first occurrence of PCR-confirmed symptomatic moderate or severe Covid-19 at least 7 days after the second study vaccination in volunteers who have not been previously infected with the virus The trial will enroll at least 25% of participants over the age of 65 and prioritize groups most affected by the Covid-19, the company said. Company's release available at (Sept. 24, 2020): https://ir.novavax.com/news-releases/news-release-details/novavax-initiates-phase-3-efficacy-trial-covid-19-vaccine-united

|
Scooped by
Juan Lama
|
Through Operation Warp Speed, the U.S. has backed seven vaccine candidates with $11 billion, securing preorders for some 800 million doses. Scientists, drugmakers and governments are moving with unprecedented haste to deliver a vaccine to protect against the new coronavirus. The fastest of them have already delivered early data from human studies, and further results from others should come quickly as the year progresses. The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world. Researchers' success or failure could determine whether the virus becomes endemic, recurring in countries around the world year after year, or is ultimately checked. With the health of their citizens at stake, governments are investing enormous sums of money into vaccine research and development, and to prepare for manufacturing and distributing what will likely need to be hundreds of millions of doses necessary to keep infection at bay. With modern-day Manhattan Projects underway, vaccines have become an issue of national security, too, raising questions of global equity and medicine access. In the U.S., the Trump administration has unveiled "Operation Warp Speed," so far pledging more than $11 billion in funding and support for seven candidates. There's no guarantee the first successful vaccine will come from the U.S., however. Some of the leading candidates are being developed overseas, with projects by the University of Oxford in the U.K. and China's CanSino Biologics the furthest along. The rest of the world might not be so lucky. "It's not like we can expect 7 billion doses the day after licensure so we can vaccinate the whole world," said Emory University vaccines expert Walter Orenstein. Yet, to truly curb circulation of the SARS-CoV-2 virus in humans, getting vaccines to nations wealthy and poor will be a vital mission. The next few months should produce a flurry of data, early answers and fresh questions, making it difficult to keep track. Here's where things stand for 13 of the most advanced, most promising or best funded vaccine candidates in the pipeline.

|
Scooped by
Juan Lama
|
The trial shows the vaccine "may be a potential winner, but efficacy and safety studies need to continue,” one expert said. A potential Covid-19 vaccine from the biotech company Novavax showed a promising immune response in a small, early trial, but not without a high rate of mostly mild side effects. The results, published Tuesday, are the latest encouraging sign in the global effort to develop a vaccine for the novel coronavirus, which has killed nearly 700,000 people around the world. But the Novavax data, much like results recently published by Moderna and AstraZeneca, are too preliminary to draw any conclusions about how well the vaccine might protect against Covid-19, experts said. “It’s a small number of people in each arm, and the study wasn’t designed to demonstrate efficacy, which are the standard caveats for a Phase 1 trial,” said Edward Belongia, an epidemiologist and vaccine researcher at the Marshfield Clinic Research Institute in Wisconsin. “Having said that, it looks very promising — at least as promising if not more so than the other vaccines we’ve looked at.” The data were published on a preprint server, meaning they have not yet been peer-reviewed. Novavax enrolled about 130 healthy volunteers in its trial and gave them either a placebo or one of four escalating doses of its vaccine. Everyone who received the vaccine developed neutralizing antibodies against SARS-CoV-2, which may help prevent infection. The best responses came from volunteers who received two injections of Novavax’s vaccine three weeks apart, plus an adjuvant meant to boost its effects. After 35 days, those participants had neutralizing antibody levels that, on average, were roughly four times higher than what was seen in a group of 32 patients who had recovered from the disease. About 80% of those volunteers had side effects at the site of injection, including pain and tenderness. More than 60% had other side effects, mostly headaches, muscle pain, and fatigue. Most reactions were mild or moderate, but eight patients had side effects that were graded severe; Novavax said none required hospitalization. All of the reactions resolved after a few days, and none was life-threatening. The study, conducted in Australia, recruited a roughly even number of men and women between the ages of 18 and 59. Volunteers were about 79% white, 15% Hispanic, 13% Asian, 6% Indigenous, and 2% Black. The median age was 31.... Preprint of the study available at medRxiv (August 4, 2020): https://www.medrxiv.org/content/10.1101/2020.08.05.20168435v1

|
Scooped by
Juan Lama
|
The Maryland-based company, which has never brought a product to market before, just made the biggest deal to date with the Trump administration’s Operation Warp Speed. The federal government will pay the vaccine maker Novavax $1.6 billion to expedite the development of 100 million doses of a coronavirus vaccine by the beginning of next year, the company said on Tuesday. The deal is the largest that the Trump administration has made so far with a company as part of Operation Warp Speed, the sprawling federal effort to make coronavirus vaccines and treatments available to the American public as quickly as possible. In doing so, the government has placed a significant bet on Novavax, a company based in Maryland that has never brought a product to market. Operation Warp Speed is a multiagency effort that seeks to carry out President Trump’s pledge to make a coronavirus vaccine available by the end of the year, but the full extent of the project is still unclear. Officials have declined to list which vaccines and treatments are part of Operation Warp Speed. In an interview on Sunday, Novavax’s president and chief executive, Stanley C. Erck, initially said he was not sure where in the government the $1.6 billion was coming from. A Novavax spokeswoman later said the money was coming from a “collaboration” between the Health and Human Services Department and the Defense Department. In May, the administration announced it was awarding up to $1.2 billion as part of Operation Warp Speed to the British drugmaker AstraZeneca, which has said that its vaccine could be available by October. Four other companies — Moderna Therapeutics, Johnson & Johnson, Merck and Sanofi — have also received federal assistance for their experimental coronavirus vaccines. “Adding Novavax’s candidate to Operation Warp Speed’s diverse portfolio of vaccines increases the odds that we will have a safe, effective vaccine as soon as the end of this year,” Alex M. Azar II, the health and human services secretary, said in a statement.
|

|
Scooped by
Juan Lama
|
Agency urged to authorize fourth U.S. vaccine despite some concern about rare heart inflammation as a side effect. A key committee of advisers to the U.S. Food and Drug Administration (FDA) today recommended nearly unanimously that the agency grant an emergency authorization to a COVID-19 vaccine from Novavax, opening the way for the first protein-based COVID-19 vaccine to become available to people in the United States. The 21-0 vote, with one abstention, marks a hard-won and long-sought milestone for the small Gaithersburg, Maryland-based biotechnology company that was moribund as the pandemic began. It found a new life as it collected $2 billion to develop a vaccine, first from an international organization that supports vaccinemaking and then from the U.S. government. “This is a case study of perseverance,” Bruce Gellin, chief of global public health strategy at the Rockefeller Foundation, said after the vote. Yet Gellin was the lone abstaining vote, saying the committee wasn’t given data on how the vaccine performs against the Omicron variants now circulating, or for how many months its protection lasts. Still, Gellin said, “This vaccine has incredible potential.” It is easy to store and transport, lasting at refrigerator temperatures for months, unlike the dominant messenger RNA (mRNA) vaccines. The thumbs up from the FDA advisers likely means the agency will allow the company to enter the U.S. market, as FDA usually follows its advisers’ lead. Novavax hopes that holdouts skeptical of mRNA vaccines and, ultimately, others seeking booster shots, will opt for its tried-and-true technology. If it wins final authorization, the vaccine will be the fourth COVID-19 jab marketed in the United States. Unlike the other three, which deliver genetic material that directs host cells to make the coronavirus’ spike protein, Novavax’s product delivers spike protein directly to recipients. Approved vaccines for shingles, hepatitis B, and influenza use similar protein-based technology. Just days before today’s meeting, the company’s stock price tumbled when FDA published data indicating that the vaccine may rarely cause myocarditis and pericarditis, a problem that has also dogged mRNA vaccines from Moderna and Pfizer-BioNTech. Perhaps in response to the volatility, NASDAQ froze trading of the stock today before the meeting opened. But 21 of the 22 advisers concluded that any risk posed by heart inflammation was outweighed by the benefits of a vaccine that showed 90.4% efficacy against early strains of SARS-CoV-2 in a trial involving 30,000 people in the United States and Mexico. The Novavax shot also has low “reactogenicity”—meaning immediate side effects, from painful arms to malaise. The committee recommended authorization for the two-shot series in adults 18 years and older. “Certainly the benefits outweigh the risks,” said adviser Michael Nelson, an allergist and immunologist at the University of Virginia School of Medicine. But in response to a query from Doran Fink, acting deputy director of FDA’s vaccine approval branch, Nelson said the agency should include a warning about heart risks in the package insert. “It would be a travesty if we didn’t mention this in the … documentation for the public to show the concern that we have.” Novavax’s vaccine, which is produced in insect cells and combined with an immune-boosting substance called an adjuvant, has already been authorized in more than 40 countries including the United Kingdom, Canada, Germany, and Australia; it has also won emergency authorizations from the European Union and the World Health Organization. But manufacturing issues have hampered Novavax’s effort and sidelined its would-be U.S. vaccinemaking facilities. In addition, the company struggled mightily to show it could make the vaccine consistently. If authorized in the United States, the product will initially be manufactured, as all Novavax vaccine currently is, by the Serum Institute of India, one of the world’s largest vaccine manufacturers. Myocarditis concerns may also dog the vaccine. The data revealed on 3 June by FDA described five cases of myocarditis that occurred in people in the vaccine arms of the Novavax clinical trials in the United States and the United Kingdom. Four occurred within 20 days of vaccination, a time frame during which there were no cases in the placebo arm. Three cases were in men ages 16 to 20. Young men have had the highest rates of myocarditis or pericarditis after receiving mRNA vaccines. “These events raise the concern for a causal association with this vaccine, similar to the association documented with mRNA COVID-19 vaccines,” FDA wrote. Novavax countered that collectively across all of its clinical trials, the risk of myocarditis and pericarditis was not significantly different in the vaccine group (0.007%) and in the placebo groups (0.005%). “We believe that the totality of the clinical evidence here is not enough to establish an overall causal relationship with the vaccine,” Denny Kim, Novavax’s chief safety officer, told the FDA advisers. But Paul Offit, a committee member and infectious disease physician at the Children’s Hospital of Philadelphia, told today’s meeting that the “handful of cases of myocarditis [that] occurred within 3 or 4 days of receiving the second dose of vaccine in young men is consistent with what was seen with the mRNA-induced myocarditis. So I think that is likely a cause and not a coincidental association.” The law governing emergency use authorizations (EUAs) by FDA requires that there is no “adequate, approved and available alternative” to a product. One advisory committee member asked Peter Marks, FDA’s top vaccine official, why the Novavax vaccine meets that requirement given that three other vaccines are already available to people in the United States. “Having a protein-based alternative may be more comfortable for some in terms of their acceptance of vaccines,” Marks replied, noting that the law “allows us some leeway” to address unmet needs. “Anything we can do to get people … to be able to accept these potentially lifesaving medical products is something that we feel we are compelled to do.” Some committee members raised eyebrows at the suggestion the Novavax vaccine would win over a substantial number of the 27 million unvaccinated Americans with its familiar technology. “I’m very skeptical that vaccine-hesitant people will elect to get this vaccine,” said committee member Jay Portnoy, an allergist and immunologist at Children’s Mercy Hospital. “Their hesitancy is more ideological than technological.” But a parade of public speakers at the meeting mostly urged the committee to authorize the vaccine. “We need to … provide options to reduce excuses,” Martha Dawson, president of the National Black Nurses Association, told the advisers. “It could be the next thing that saves your life or your loved one’s life.” When FDA’s advisers gave a green light to the Moderna and Pfizer vaccines in late 2020, the agency acted quickly to issue EUAs for those vaccines. But the Novavax process may not move so speedily: FDA says it needs additional manufacturing and product information before an EUA can be issued. The U.S. Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices would also need to make a recommendation for who should receive the vaccine, and that committee has not yet scheduled a meeting. Science (June 7, 2022): https://doi.org/10.1126/science.add3781

|
Scooped by
Juan Lama
|
BACKGROUND Early clinical data from studies of the NVX-CoV2373 vaccine (Novavax), a recombinant nanoparticle vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that contains the full-length spike glycoprotein of the prototype strain plus Matrix-M adjuvant, showed that the vaccine was safe and associated with a robust immune response in healthy adult participants. Additional data were needed regarding the efficacy, immunogenicity, and safety of this vaccine in a larger population. METHODS In this phase 3, randomized, observer-blinded, placebo-controlled trial conducted at 33 sites in the United Kingdom, we assigned adults between the ages of 18 and 84 years in a 1:1 ratio to receive two intramuscular 5-μg doses of NVX-CoV2373 or placebo administered 21 days apart. The primary efficacy end point was virologically confirmed mild, moderate, or severe SARS-CoV-2 infection with an onset at least 7 days after the second injection in participants who were serologically negative at baseline. RESULTS A total of 15,187 participants underwent randomization, and 14,039 were included in the per-protocol efficacy population. Of the participants, 27.9% were 65 years of age or older, and 44.6% had coexisting illnesses. Infections were reported in 10 participants in the vaccine group and in 96 in the placebo group, with a symptom onset of at least 7 days after the second injection, for a vaccine efficacy of 89.7% (95% confidence interval [CI], 80.2 to 94.6). No hospitalizations or deaths were reported among the 10 cases in the vaccine group. Five cases of severe infection were reported, all of which were in the placebo group. A post hoc analysis showed an efficacy of 86.3% (95% CI, 71.3 to 93.5) against the B.1.1.7 (or alpha) variant and 96.4% (95% CI, 73.8 to 99.5) against non-B.1.1.7 variants. Reactogenicity was generally mild and transient. The incidence of serious adverse events was low and similar in the two groups. CONCLUSIONS A two-dose regimen of the NVX-CoV2373 vaccine administered to adult participants conferred 89.7% protection against SARS-CoV-2 infection and showed high efficacy against the B.1.1.7 variant. (Funded by Novavax; EudraCT number, 2020-004123-16. opens in new tab.) Published in NEJM (June 30, 2021): https://doi.org/10.1056/NEJMoa2107659

|
Scooped by
Juan Lama
|
GAITHERSBURG, Md., June 11, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company developing next-generation vaccines for serious infectious diseases, today announced preclinical and clinical data on the company's original recombinant protein COVID-19 vaccine candidate, NVX-CoV2373, and for a new vaccine directed against the SARS-CoV-2 Beta (B.1.351) variant, which was originally identified in South Africa. The data show that the vaccines demonstrated strong immunogenicity and protection against both the Alpha (B.1.1.7) variant, which was originally identified in the United Kingdom, and the Beta (B.1.351) variant as well as the original SARS-CoV-2 in animal and human studies. A preprint of the manuscript, 'Immunogenicity and In vivo protection of a variant nanoparticle vaccine that confers broad protection against emerging SARS-CoV-2 variants,' is available at bioRxiv.org and has been submitted for peer review. "While current vaccines are effective against selected SARS-CoV-2 variant strains, newly emerging variants are also being identified that have the ability to overcome vaccine induced immunity," said Matthew Frieman, PhD, Associate Professor of Microbiology and Immunology at the University of Maryland School of Medicine, who collaborated on these studies. "This work demonstrates that variant vaccines that protect against these newly emerging variants have the potential to be highly effective and may produce broader protection against variants we know of and those that will arise in the future. Clinical trials will provide further evidence on the effectiveness of variant vaccines." The studies compared the Beta (B.1.351)-directed vaccine to Novavax' prototype vaccine candidate as standalone, in combination, and as heterologous prime boost vaccine. The findings show a broad array of cellular and humoral responses in animal models against all virus strains evaluated. The Alpha (B.1.1.7) and Beta (B.1.351) variant strains have created public health concerns due to increased transmission rates and lower efficacy of current vaccines seen against Beta (B.1.351). "These data suggest that not only could one booster dose of this variant-directed vaccine potentially provide a robust, protective immune boost after vaccination against the original SARS-CoV-2 virus, but also the potential to provide broad protection against various virus strains if used as a primary vaccine regimen," said Gregory M. Glenn, M.D., President of Research and Development, Novavax. "This broad immune coverage is vital to controlling the pandemic as variants of concern continue to emerge worldwide that could jeopardize the protection created through ongoing COVID-19 vaccination efforts." Study 1: Immunogenicity and Protection in Mice Mice were immunized with NVX-CoV2373 or rS-B.1.351 alone, in combination, or as a heterologous prime boost. Mice vaccinated with any of the four regimens displayed elevated antibody titers against both the original and Beta (B.1.351) spike. Heterologous or bivalent vaccination is highly immunogenic against the prototype spike compared to monovalent approaches, while immunization with monovalent rS-B.1.351 or bivalent spike resulted in the highest anti-B.1.351 spike IgG titers among regimens tested. Additionally, mice immunized with rS-B.1.351 alone produced elevated neutralizing antibody titers to the Alpha (B.1.1.7) and Beta (B.1.351) strains compared to titers upon immunization with the prototype-directed strain. Titers were similar in the heterologous and bivalent vaccine groups. Antibodies produced after vaccination with rS-B.1.351 inhibited binding between human angiotensin converting enzyme-2 receptor (hACE2) and the variant spike or original spike to the same degree. This indicates that rS-B.1.351 can efficiently protect "backward" against original SARS-CoV-2 strains. In contrast, NVX-CoV2373 was less efficient at protecting "forward" against the Beta (B.1.351) variant strain. Whether immunized with prototype vaccine or rS-B.1.351 alone, in combination, or as a heterologous prime boost, mice were protected when challenged with live Alpha (B.1.1.7) or Beta (B.1.351) variant strains of SARS-CoV-2. Study 2: Anamnestic Response in Baboons A cohort of baboons that were originally immunized with prototype vaccine were boosted approximately one year later with one or two doses of rS-B.1.351. Seven days after the first rS-B.1.351 boost, those that originally received adjuvanted prototype-directed vaccine exhibited a strong immune response, with anti-Spike IgG titers that were higher than the peak immune response observed during the primary immunization series. The rS-B.1.351 boost elicited comparable antibody titers against original and rS-B.1.351 spike. Further, the same cohort exhibited a strong neutralizing response to Alpha (B.1.1.7) and Beta (B.1.351) strains and hACE2-inhibiting antibody response following the boost, despite having undetectable titers before the boost. High neutralizing antibody titers were observed seven days post-boost, demonstrating "a robust, durable antibody response even one year after the primary vaccination series." These results suggest that one dose of a variant-directed vaccine may be sufficient for boosting regimens after previous immunization with a COVID-19 vaccine based on the original spike strain. Study 3: Robust Antibody Response in Humans NVX-CoV2373 is currently being studied in multiple clinical trials, including in locations where Alpha (B.1.1.7) and Beta (B.1.351) variant strains are widespread. Thirty randomly selected human serum samples from Phase 2 clinical trial participants after their second dose of the vaccine were assayed. The sera were analyzed for their ability to neutralize Alpha (B.1.1.7) and Beta (B.1.351) strains. The trial participants' sera demonstrated a neutralizing capacity of the Alpha (B.1.1.7) strain equal to NVX-CoV2373, with a modest reduction in neutralizing capacity against the Beta (B.1.351) strain. These data support the development and production of a Beta (B.1.351) targeted vaccine, which also demonstrated to be efficient at protecting mice against Beta (B.1.351) and to induce a strong response in primates originally immunized with NVX-CoV2373. Furthermore, the data support that a booster vaccine containing a variant strain could both increase antibody levels as well as broaden coverage. Novavax expects to initiate further clinical testing of rS-B.1.351 in the fall of 2021.... See bioRxiv (June 9, 2021): https://doi.org/10.1101/2021.06.08.447631

|
Scooped by
Juan Lama
|
Novavax Inc's COVID-19 vaccine was 96% effective in preventing cases caused by the original version of the coronavirus in a late-stage trial conducted in the United Kingdom, the company said on Thursday, moving it a step closer to regulatory approval. There were no cases of severe illness or deaths among those who got the vaccine, the company said, in a sign that it could stop the worse effects of new variants that have cropped up. The vaccine was 86% effective in protecting against the more contagious virus variant first discovered and now prevalent in the United Kingdom, for a combined 90% effectiveness rate overall based on data from infections of both versions of the coronavirus. Novavax shares jumped 22% in after-hours trading to $229. They were trading below $10 on Jan. 21, 2020, when the company announced it was developing a coronavirus vaccine. In a smaller trial conducted in South Africa - where volunteers were primarily exposed to another newer, more contagious variant widely circulating there and spreading around the world - the Novavax vaccine was 55% effective, based on people without HIV, but still fully prevented severe illness. Novavax Chief Medical Officer Filip Dubovsky said the performance in South Africa suggests there may still be a case for using it in areas where the South African variant is dominant. Novavax is also developing new formulations of its vaccine to protect against emerging variants and plans to initiate clinical testing of these shots in the second quarter of this year. Results from the final analysis of the UK trial were largely in line with interim data released in January. The company expects to use the data to submit for regulatory authorization in various countries. It is not clear when it will seek U.S. authorization or if regulators will require it to complete an ongoing trial in the United States. Novavax expects data from a 30,000-person trial in the United States and Mexico by early April. Dubovsky said that Novavax is still planning to file for authorization from UK regulators early in the second quarter of 2021. The UK trial, which enrolled more than 15,000 people aged 18 to 84, assessed efficacy of the vaccine during a period with high transmission of the UK virus variant now circulating widely. The shot’s effectiveness in the South Africa trial declined to around 49% when the analysis included data from HIV-positive participants. The vaccine could be cleared for use in the United States as soon as May if U.S. regulators decide the UK data is enough to make a decision. It could take a couple months longer if they insist on first seeing data from the U.S. trial, its chief executive told Reuters earlier this month. “Ultimately, they have to decide whether the data we can bring to the table is adequate or whether they would prefer to wait on data from our U.S. study,” Dubovsky said on Thursday. Novavax’s vaccine production plants should all be fully functional by April, executives said on a March investor call. The drugmaker expects to have tens of millions of doses stockpiled and ready to ship in the United States when it receives authorization, CEO Stanley Erck told Reuters. Novavax plans to produce its two-shot vaccine at eight manufacturing locations, including the Serum Institute of India. If authorized, it would follow three COVID-19 vaccines previously approved for use in Britain from Pfizer and partner BioNTech, Moderna Inc and the AstraZeneca shot developed with Oxford University. The Maryland-based company has received $1.6 billion from the U.S. government in funding for the vaccine trial and to secure 100 million doses. Company's Press Release (March 11, 2021):

|
Scooped by
Juan Lama
|
An early analysis in Britain found that the vaccine had an efficacy rate of nearly 90 percent. But in a small South Africa trial, the efficacy rate dropped to just under 50 percent. Novavax, a little-known company supported by the U.S. federal government’s Operation Warp Speed, said for the first time on Thursday that its Covid-19 vaccine offered robust protection against the virus. But it also found that the vaccine is not as effective against the fast-spreading variant first discovered in South Africa, another setback in the global race to end a pandemic that has already killed more than 2.1 million people. That could be a problem for the United States, which hours earlier reported its first known cases of the contagious variant in two unrelated people in South Carolina. And it came just days after Moderna and Pfizer said that their vaccines were also less effective against the same variant. Novavax, which makes one of six vaccine candidates supported by Operation Warp Speed last summer, has been running trials in Britain, South Africa, the United States and Mexico. It said Thursday that an early analysis of its 15,000-person trial in Britain revealed that the two-dose vaccine had an efficacy rate of nearly 90 percent there. But in a small trial in South Africa, the efficacy rate dropped to just under 50 percent. Almost all the cases that scientists have analyzed there so far were caused by the variant, known as B.1.351. The data also showed that many trial participants were infected with the variant even after they had already had Covid. “We have the first trial — we are the first to conduct an efficacy trial — in the face of a changing virus,” said Stanley Erck, the president and chief executive of Novavax. He said that researchers expected the variants could change the trial results, but “the amount of change has been a bit of a surprise to everyone.” The South Africa trial was relatively small — with just 4,400 volunteers — and was not designed to come up with a precise estimate of how much protection the vaccine provides. Still, the results were striking enough that the company said it would soon begin testing a new vaccine tailored to protect against the variant from South Africa. “You’re going to have to make new vaccines,” Mr. Erck said. John Moore, a virologist at Weill Cornell Medicine who was not involved in the studies, praised the results. “Fifty percent is not as good as 100, but it’s a damn sight better than zero,” he said, noting that given the strong results in Britain, it was likely very similar in efficacy to the Pfizer and Moderna vaccines. While the Pfizer and Moderna vaccines rely on a newer mRNA technology that has not been used in previous vaccines, Novavax’s candidate employs an older, more established method that relies on injecting coronavirus proteins to provoke an immune response. The fact that three vaccines all appeared to show lowered effectiveness against the variant from South Africa is not encouraging, and the results Novavax announced Thursday were the first to occur outside of a laboratory, testing how well a vaccine worked in people infected with a new variant. Johnson & Johnson is also on the cusp of announcing results of its Covid-19 vaccine trials, and has also tested its candidate in South Africa. The announcement from Novavax raises the stakes for Johnson & Johnson. The company was expected to announce its results as early as last weekend, and the delay has triggered speculation among scientists that the firm has also discovered that its vaccine worked less well in South African trial volunteers who were infected with the variant. In an earnings call on Tuesday, Alex Gorsky, the chief executive officer of the company, said they were looking forward to sharing results from their late-stage trial by early next week. The emergence of several highly contagious variants has complicated efforts to bring the pandemic under control, leading world leaders to shut down travel to places like Britain and South Africa even as the variants already appear to have circled the globe. In the United States, researchers have warned that the variant first identified in Britain, which is believed to be more infectious, could become the dominant form of the virus in this country by March. The United States is well behind other countries in testing for such variants, and the one from South Africa has been found in about 30 countries. But experts have also said there are reasons for optimism, noting that the vaccines remain effective. The best way to combat contagious new variants is to continue vaccination and other public health measures, which will slow the virus’s ability to infect new people and mutate further. “This is really worrisome,” said Dr. Peter Hotez, a vaccine expert at the Baylor College of Medicine and the inventor of a coronavirus vaccine. “We have to have the American people vaccinated by late in the spring or early summer to have any hope in preventing the South African and the U.K. variants from taking over.” Drug makers could update their vaccines and offer new shots at regular intervals, similar to the flu vaccine....... Novavax Press Release (Jan. 28, 2021): https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3

|
Scooped by
Juan Lama
|
Novavax Inc on Tuesday delayed the start of a late-stage U.S. trial of its experimental coronavirus vaccine by roughly a month to the end of November, citing delays in scaling up the manufacturing process. The U.S.-based drug developer said data from a separate Phase III trial being conducted in Britain was expected by the first quarter of 2021 and could be the basis for global regulatory approvals although it did not elaborate. Shares of the company rose nearly 3%. It is not immediately clear whether that could apply in the United States. Novavax did not respond to a request for clarification. “I think the FDA has generally been loathe to approve vaccines for Americans that haven’t been tested in Americans, historically,” Dr. Paul Offit, an infectious disease expert at the University of Pennsylvania and a member of the U.S. Food and Drug Administration’s vaccine advisory panel, said on in an interview with the editor of JAMA medical journal on Tuesday. Data from an early-to-mid stage trial of the vaccine is expected on Friday, the company said. Earlier data had showed the vaccine produced high levels of antibodies against the novel coronavirus. A handful of companies, including larger rivals Pfizer Inc and AstraZeneca Plc, are conducting late-stage trials of their experimental COVID-19 vaccines, though none have reported pivotal data that would be used to seek emergency authorization or approval. The companies, including Novavax, have already made distribution deals with several countries for the vaccines, once approved. Novavax in August said it will supply 60 million doses of its coronavirus vaccine to the UK from as early as the first quarter of 2021. The company is also preparing to deliver 100 million doses to the United States by January after it was awarded $1.6 billion for its potential vaccine, and has also signed supply agreements with Canada and Japan.

|
Scooped by
Juan Lama
|
The companies hope to earn the trust of the public and of scientists who have clamored for details of the studies. Two drug companies that are leading the race to develop coronavirus vaccines bowed to public pressure on Thursday, abandoning their traditional secrecy and releasing comprehensive road maps of how they are evaluating their vaccines. The companies, Moderna and Pfizer, revealed details about how participants are being selected and monitored, the conditions under which the trials could be stopped early if there were problems, and the evidence researchers will use to determine whether people who got the vaccines were protected from Covid-1. Moderna’s study will involve 30,000 participants, and Pfizer’s 44,000. Companies typically share these documents after their studies are complete. The disclosures while the trials are still underway, a rare move, are aimed at addressing growing suspicion among Americans that President Trump’s drive to produce a vaccine before the election on Nov. 3 could result in a product that was unsafe. The plan released by Moderna on Thursday morning included a likely timetable that could reach into next year for determining whether its vaccine works. It does not jibe with the president’s optimistic predictions of a vaccine widely available to the public in October. Pfizer’s plan does not appear to estimate when its results could be available. Its chief executive has said repeatedly that the company hopes to have an answer as early as October. Moderna has said only that it could have a result before the end of the year. Moderna’s 135-page plan, or protocol, indicated that the company’s first analysis of early trial data might not be conducted until late December, though company officials now say they expect the initial analysis in November. In any case, there may not be enough information then to determine whether the vaccine works, and the final analysis might not take place until months later, heading into the spring of next year. Moderna’s timeline meshes with the cautionary estimates from many researchers, including Dr. Robert R. Redfield, the director of the Centers for Disease Control and Prevention, who told senators on Wednesday that a vaccine would not be widely available until the middle of next year. Hours later, Mr. Trump sharply contradicted him, making unsubstantiated projections that a vaccine could become widely available weeks from now. On Wednesday, Joseph R. Biden Jr., the Democratic presidential nominee, said in Wilmington, Del., that the process used to evaluate and approve a vaccine would have to be “totally transparent” to win public confidence. He has said that Mr. Trump’s calls for companies and regulators to speed the process have shaken the public’s faith in vaccines and that politics has no place in vaccine development. Researchers in particular have been urging vaccine makers to share the detailed blueprints of their studies so that outside experts can evaluate them. At least one expert, after reading the plans, has already raised questions about the way the trials were designed. “I want to acknowledge a good deed done,” said Peter Doshi, who is on the faculty at the University of Maryland School of Pharmacy in Baltimore and an editor with The BMJ, a medical journal. He previously requested the plans from Moderna and Pfizer. “They have opened up, for the first time, the ability for researchers not involved in the trial to form their own independent judgment about the design of this study.” Until now, none of the nine companies that are testing vaccines in large clinical trials had released this level of detail. Moderna, AstraZeneca and Pfizer, which is collaborating with the German company BioNTech, are among the front-runners in the global race to produce a vaccine to fight the pandemic. A spokeswoman for AstraZeneca said the company intended to publish its protocol shortly. Novavax, which is expected to start a large, advanced clinical trial later this year, also did not comment. Johnson & Johnson, which has said it plans to begin a large trial this month, said it would have “more information to share” when the trial starts.AstraZeneca’s trial was stopped temporarily because of serious illness in a participant. It has resumed in Britain and Brazil, but not in the United States. Earlier studies of both vaccines in small numbers of people found that after the second shot, they developed so-called neutralizing antibodies, which can inactivate the virus in lab tests. The vaccines also produced a favorable response involving T-cells, another part of the immune system...

|
Scooped by
Juan Lama
|
Although none of the coronavirus vaccines under development has proved its efficacy yet in clinical trials, at least 5.7 billion doses have been pre-ordered around the world. First shipments of a COVID-19 vaccine created by Western laboratories have often been snapped up by the United States. Five vaccines — three Western and two Chinese — are in Phase 3 efficacy trials involving thousands of people. In a surprise announcement, Russian President Vladimir Putin claimed Tuesday that a vaccine dubbed Sputnik V — after the Soviet satellite — conferred “sustainable immunity” against the novel coronavirus. As research laboratories around the world race to develop a vaccine, manufacturers have received financing to help them prepare to have millions of doses ready to administer in 2021 or even before the end of the year. Oxford University, working with the Swedish-British pharmaceutical group AstraZeneca, hopes to have results by September, while the US biotech company Moderna, partnering with the U.S. National Institutes of Health (NIH), is aiming for the end of the year, possibly November. U.S. President Donald Trump has launched “Operation Warp Speed” in a bid to develop, manufacture and distribute a COVID-19 vaccine to all Americans by January 2021. Hundreds of millions of dollars have been directed to vaccine developers, including nearly $500 million to Johnson & Johnson at the end of March. The United States has allocated funding to more companies than any other nation in the hope that one of them will come up with the vaccine to counter the highly contagious virus. So far, Washington has handed out at a total of at least $9.4 billion to seven vaccine developers and signed manufacturing contracts with five of them to provide 700 million doses. The companies involved are: Johnson & Johnson, Moderna, Oxford/AztraZeneca, Novavax, Pfizer/BioNTech, Sanofi/GSK, Merck Sharp and Dohme. Two vaccine developers — Oxford/AztraZeneca and Sanofi/GSK — have signed or are in advanced negotiations with the European Commission to provide a combined 700 million vaccine doses. Britain, because of Brexit, is negotiating a separate pre-order of 250 million doses from four developers. Japan is counting on 490 million doses from three suppliers, including 250 million from Novavax of the United States. Pharmaceutical giant Takeda bought the rights to a Novavax vaccine for Japan, which has funded the research. It would be produced locally. Brazil chose a similar model, ordering 100 million doses from AstraZeneca and partnering with China’s Sinovac to produce 120 millions of CoronaVac, which is already undergoing testing with Brazilians. Clinical tests of two Chinese vaccine candidates — Sinovac and Sinopharm — are well underway but only a few international partnerships have been announced, the one with Brazil and a possible one with Indonesia. Russia said 20 nations have pre-ordered 1 billion doses of Sputnik V and that with foreign partners it would be able to produce 500 million doses a year in five countries. The Coalition for Epidemic Preparedness Innovations, launched in 2017 by Norway, India, the Bill and Melinda Gates Foundation and the Wellcome Trust, seeks to ensure that there is “equitable access” to future vaccines. It has pre-ordered 300 million doses from AstraZeneca for dozens of developing countries in a partnership with The Vaccine Alliance. Billions of doses would be produced for Asia and elsewhere by the giant Serum Institute of India (SII), the largest vaccine producer in the world. Novavax and AstraZeneca have separately signed agreements with SII to produce a billion doses each for India and low- and middle-income countries on the condition that they prove their efficacy in clinical trials.

|
Scooped by
Juan Lama
|
Novavax just received the Trump administration’s largest vaccine contract. In the Maryland company’s 33-year history, it has never brought a vaccine to market. In late February, as the coronavirus spread around the world, Dr. Richard Hatchett, the head of an international nonprofit that gives money to vaccine developers, got on an important call to discuss vaccine candidates after his plane touched down at London’s Heathrow Airport. Executives from the Bill & Melinda Gates Foundation, which helped found and finance the nonprofit, were on the line, enthusiastic about Novavax, a small biotech company they thought had the potential to develop a vaccine against the virus — fast. Although the company, based in Gaithersburg, Md., had never brought a vaccine to market in its 33-year history, these experts were optimistic about its technology, which uses moth cells to pump out crucial molecules at a much faster rate than typical vaccines — a major advantage in a pandemic. Dr. Hatchett’s organization, the Coalition for Epidemic Preparedness Innovations, would go on to invest $388 million in the company’s coronavirus vaccine. With that powerful backing, Novavax made an aggressive push to the U.S. government. The company’s effort paid off last week when Operation Warp Speed, the Trump administration’s effort to hurry coronavirus vaccines to the market, gave Novavax $1.6 billion, the largest award to date. The company’s stock surged 30 percent. It was a dramatic turnaround for a little-known company that, just one year earlier, had been on the verge of collapse. One of its leading vaccine candidates — to prevent a deadly virus in infants — had failed for the second time in three years. The company’s stock was trading so low that it risked being removed from the Nasdaq. Looking for cash, it sold its manufacturing facilities. Word spread around the small world of Maryland biotech that Novavax might be closing soon. Novavax’s good fortune may appear puzzling, given its track record and the air of secrecy surrounding Operation Warp Speed. But for those in the insular biotech world where connections matter, it is far less surprising. In the face of a deadly pandemic that is devastating the economy, the government is placing huge bets on vaccines and treatments that could enable a return to some semblance of normal life....
|



 Your new post is loading...
Your new post is loading...
























Buy Klonopin without prescription
buy liquid morphine online
Buy Morphine online uk
Buy Norco Online Without Prescription
Buy Oramorph without prescription
Buy oxycontin online uk
Buy Quaaludes Online
buy sildenafil 50 mg tablet
Buy yellow Xanax online
Dilaudid for pain
Order Acxion phentermine 30mg
Order codeine without prescription