Your new post is loading...

|
Scooped by
Juan Lama
|
In November 2023, SARS-CoV-2 XBB descendants, including EG.5.1 (XBB.1.9.2.5.1), the currently predominant lineage, are circulating worldwide according to Nextstrain. EG.5.1 has a characteristic amino acid substitution in the spike protein (S), S:F456L, which contributes to its escape from humoral immunity. EG.5.1 has further evolved, and its descendant lineage harboring S:L455F (i.e., EG.5.1+S:L455F) emerged and was named HK.3 (XBB.1.9.2.5.1.1.3). HK.3 was initially discovered in East Asia and is rapidly spreading worldwide. Notably, the XBB subvariants bearing both S:L455F and S:F456L substitutions, including HK.3, are called the FLip variants. These FLip variants, such as JG.3 (XBB.1.9.2.5.1.3.3), JF.1 (XBB.1.16.6.1) and GK.3 (XBB.1.5.70.3), have emerged convergently, suggesting that the acquisition of these two substitutions confers a growth advantage to XBB in the human population. Here, we investigated the virological properties of HK.3 as a representative of the FLip variants. Preprint in bioRxiv (Nov. 15, 2023): https://doi.org/10.1101/2023.11.14.566985

|
Scooped by
Juan Lama
|
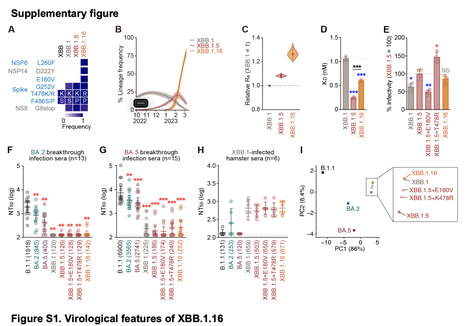
At the end of March 2023, XBB.1.16, a SARS-CoV-2 omicron XBB subvariant, emerged and was detected in various countries. Compared to XBB.1.5, XBB.1.16 has two substitutions in the S protein: E180V is in the N-terminal domain, and T478K in the receptor-binding domain (RBD). We first show that XBB.1.16 had an effective reproductive number (Re) that was 1.27- and 1.17-fold higher than the parental XBB.1 and XBB.1.5, respectively, suggesting that XBB.1.16 will spread worldwide in the near future. In fact, the WHO classified XBB.1.16 as a variant under monitoring on March 30, 2023. Neutralization assays demonstrated the robust resistance of XBB.1.16 to breakthrough infection sera of BA.2 (18-fold versus B.1.1) and BA.5 (37-fold versus B.1.1). We then used six clinically-available monoclonal antibodies and showed that only sotrovimab exhibits antiviral activity against XBB subvariants, including XBB.1.16. Our results suggest that, similar to XBB.1 and XBB.1.5, XBB.1.16 is robustly resistant to a variety of anti-SARS-CoV-2 antibodies. Our multiscale investigations suggest that XBB.1.16 that XBB.1.16 has a greater growth advantage in the human population compared to XBB.1 and XBB.1.5, while the ability of XBB.1.16 to exhibit profound immune evasion is comparable to XBB.1 and XBB.1.5. The increased fitness of XBB.1.16 may be due to (1) different antigenicity than XBB.1.5; and/or (2) the mutations in the non-S viral protein(s) that may contribute to increased viral growth efficiency. Published in bioRxiv (april 6, 2023): https://doi.org/10.1101/2023.04.06.535883

|
Scooped by
Juan Lama
|
BF.7 is a new version of the coronavirus SARS-CoV-2 that's driving a surge of infection in China. Since the COVID variant omicron emerged in late 2021, it has rapidly evolved into multiple subvariants. One subvariant, BF.7, has recently been identified as the main variant spreading in Beijing, and is contributing to a wider surge of COVID infections in China. But what is this new variant, and should we be worried? Although reports from China about this variant's characteristics are concerning, it doesn't appear to be growing too much elsewhere in the world. Here's what we know. BF.7, short for BA.5.2.1.7, is a sub-lineage of the omicron variant BA.5. Reports from China indicate BF.7 has the strongest infection ability out of the omicron subvariants in the country, being quicker to transmit than other variants, having a shorter incubation period, and with greater capacity to infect people who have had a previous COVID infection, or been vaccinated, or both. To put this into context, BF.7 is believed to have an R0, or basic reproduction number, of 10 to 18.6. This means an infected person will transmit the virus to an average of 10 to 18.6 other people. Research has shown omicron has an average R0 of 5.08. The high transmission rate of BF.7, taken with the risk of hidden spread due to the many asymptomatic carriers, is understood to be causing significant difficulty in controlling the epidemic in China. The symptoms of an infection with BF.7 are similar to those associated with other omicron subvariants, primarily upper respiratory symptoms. Patients may have a fever, cough, sore throat, runny nose and fatigue, among other symptoms. A minority of people can also experience gastrointestinal symptoms like vomiting and diarrhoea. BF.7 may well cause more serious illness in people with weaker immune systems. As omicron has evolved, we've seen the emergence of new subvariants better able to escape immunity from vaccination or prior infection. BF.7 is no different. BF.7 carries a specific mutation, R346T, in the spike protein of SARS-CoV-2 (a protein on the surface of the virus that allows it to attach to and infect our cells). This mutation, which we also see in BF.7's "parent" variant BA.5, has been linked with enhancing the capacity of the virus to escape neutralising antibodies generated by vaccines or previous infection. A recent study examined the neutralisation of BF.7 in sera (a component of blood that should contain antibodies) from triple-vaccinated healthcare workers, as well as patients infected during the omicron BA.1 and BA.5 waves of the pandemic. BF.7 was resistant to neutralisation, driven partly by the R346T mutation. BF.7 has been detected in several other countries around the world including India, the U.S., the U.K. and several European countries such as Belgium, Germany, France and Denmark. Despite BF.7's immune-evasive characteristics, and worrying signs about its growth in China, the variant seems to be remaining fairly steady elsewhere. For example, in the U.S. it was estimated to account for 5.7% of infections up to December 10, down from 6.6% the week prior. While the U.K. Health Security Agency identified BF.7 as one of the most concerning variants in terms of both growth and neutralisation data in a technical briefing published in October (it accounted for over 7% of cases at that time), the most recent briefing says BF.7 has been de-escalated due to reduced incidence and low growth rates in the U.K. We don't know exactly why the situation looks different in China. BF.7's high R0 might be due in part to a low level of immunity in the Chinese population from previous infection, and possibly vaccination too. We should, of course, be cautious about the data from China as it's based on reports, not peer-reviewed evidence yet. Since the emergence of SARS-CoV-2 three years ago, the virus has continued to evolve, acquiring genetic mutations more rapidly than expected. The emergence of BF.7 and other new variants is concerning. But vaccination is still the best weapon we have to fight COVID. And the recent approval by the U.K. drugs regulator of bivalent boosters, which target omicron alongside the original strain of SARS-CoV-2, is very promising. This article is republished from The Conversation under a Creative Commons license. Read the original article.

|
Scooped by
Juan Lama
|
The newly emerged SARS-CoV-2 Omicron BQ.1.1, XBB.1, and other sublineages have accumulated additional spike mutations that may affect vaccine effectiveness. Here we report neutralizing activities of three human serum panels collected from individuals 1-3 months after dose 4 of parental mRNA vaccine (post-dose-4), 1 month after a BA.5-bivalent-booster (BA.5-bivalent-booster), or 1 month after a BA.5-bivalent-booster with previous SARS-CoV-2 infection (BA.5-bivalent-booster-infection). Post-dose-4 sera neutralized USA-WA1/2020, BA.5, BF.7, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 SARS-CoV-2 with geometric mean titers (GMTs) of 1533, 95, 69, 62, 26, 22, and 15, respectively; BA.5-bivalent-booster sera improved the GMTs to 3620, 298, 305, 183, 98, 73, and 35; BA.5-bivalent-booster-infection sera further increased the GMTs to 5776, 1558,1223, 744, 367, 267, and 103. Thus, although BA.5-bivalent-booster elicits better neutralization than parental vaccine, it does not produce robust neutralization against the newly emerged Omicron BA.2.75.2, BQ.1.1, and XBB.1. Previous infection enhances the magnitude and breadth of BA.5-bivalent-booster-elicited neutralization. Preprint in bioRxiv (Nov. 02, 2022): https://doi.org/10.1101/2022.10.31.514580

|
Scooped by
Juan Lama
|
Background: Epidemiological evidence for immune imprinting was investigated in immune histories related to vaccination in Qatar from onset of the omicron wave, on December 19, 2021, through September 15, 2022. Methods: Matched, retrospective, cohort studies were conducted to investigate differences in incidence of SARS-CoV-2 reinfection in the national cohort of persons who had a primary omicron infection, but different vaccination histories. History of primary-series (two-dose) vaccination was compared to that of no vaccination, history of booster (three-dose) vaccination was compared to that of two-dose vaccination, and history of booster vaccination was compared to that of no vaccination. Associations were estimated using Cox proportional-hazards regression models. Results: The adjusted hazard ratio comparing incidence of reinfection in the two-dose cohort to that in the unvaccinated cohort was 0.43 (95% CI: 0.38-0.48). The adjusted hazard ratio comparing incidence of reinfection in the three-dose cohort to that in the two-dose cohort was 1.38 (95% CI: 1.16-1.65). The adjusted hazard ratio comparing incidence of reinfection in the three-dose cohort to that in the unvaccinated cohort was 0.53 (95% CI: 0.44-0.63). All adjusted hazard ratios appeared stable over 6 months of follow-up. Divergence in cumulative incidence curves in all comparisons increased markedly when incidence was dominated by BA.4/BA.5 and BA.2.75*. No reinfection in any cohort progressed to severe, critical, or fatal COVID-19. Conclusions: History of primary-series vaccination enhanced immune protection against omicron reinfection, but history of booster vaccination compromised protection against omicron reinfection. These findings do not undermine the short-term public health utility of booster vaccination. Preprint in medRxiv (Nov. 1, 2022): https://doi.org/10.1101/2022.10.31.22281756

|
Scooped by
Juan Lama
|
The BA.2.75* sublineage of SARS-CoV-2 B.1.1.529 (omicron) variant escapes neutralizing antibodies. We estimated effectiveness of prior infection in preventing reinfection with BA.2.75* using a test-negative, case-control study design. Effectiveness of prior pre-omicron infection against BA.2.75* reinfection, irrespective of symptoms, was 6.0% (95% CI: 1.5-10.4%). Effectiveness of prior BA.1/BA.2 infection was 49.9% (95% CI: 47.6-52.1%) and of prior BA.4/BA.5 infection was 80.6% (95% CI: 71.2-87.0). Effectiveness of prior pre-omicron infection followed by BA.1/BA.2 infection against BA.2.75* reinfection was 56.4% (95% CI: 50.5-61.6). Effectiveness of prior pre-omicron infection followed by BA.4/BA.5 infection was 91.6% (95% CI: 65.1-98.0). Analyses stratified by time since prior infection indicated waning of protection since prior infection. Analyses stratified by vaccination status indicated that protection from prior infection is higher among those vaccinated, particularly among those who combined index-virus-type vaccination with a prior omicron infection. A combination of pre-omicron and omicron immunity is most protective against BA.2.75* reinfection. Viral immune evasion may have accelerated recently to overcome high immunity in the global population, thereby also accelerating waning of natural immunity. Preprint available in medRxiv (Oct. 30, 2022): https://doi.org/10.1101/2022.10.29.22281606

|
Scooped by
Juan Lama
|
President Biden cautioned that the immunocompromised are at heightened risk this winter and should talk to their physician about precautions. - Omicron subvariants are reducing the effectiveness of antibody treatments that have played a crucial role in keeping people with weak immune systems safe.
- President Joe Biden cautioned the immunocompromised that they are at heightened risk this winter and should talk to their physician about what precautions to take.
- Dr. Ashish Jha, head of the White House Covid task force, said treatment options are dwindling for the vulnerable because Congress failed to pass more funding.
Emerging omicron subvariants are resistant to key antibody treatments for HIV patients, kidney transplant recipients and other immunocompromised people, making them particularly vulnerable to Covid this winter, the White House warned this week. “With some of the new subvariants that are emerging, some of the main tools we’ve had to protect the immunocompromised like Evusheld may not work moving forward. And that’s a huge challenge,” Dr. Ashish Jha, head of the White House Covid task force, told reporters on Tuesday. President Joe Biden on Tuesday cautioned the estimated 7 million adults in the U.S. who have compromised immune systems that they are particularly at risk, but he could offer little in the way of reassurance other than telling them to consult their physician about what precautions to take. “New variants may make some existing protections ineffective for the immunocompromised,” the president said before getting his booster Tuesday. “Sadly, this means you may be at a special risk this winter. I urge you to consult your doctors on the right steps to protect yourself, take extra precautions.” The message clashes with repeated White House assurances that the U.S. has all the vaccines and treatments it needs to fight Covid this winter as public health officials are expecting another surge. While this may be true for the general population, it is not the case for people with weak immune systems. They include those with cancer, those who have had organ transplants, people living with HIV and individuals who are taking medicine for autoimmune diseases. Evusheld is an antibody cocktail authorized by the Food and Drug Administration to prevent Covid in people ages 12 and older who have moderately or severely compromised immune systems. The drug is administered as two injections, prior to infection, every six months. Evusheld, made by AstraZeneca, has helped fill a gap in protection for those with weak immune systems who cannot mount a strong response to the vaccines. The drug, plus several rounds of vaccination, has led to significant declines in hospitalization among this cohort over the past several months, according Camille Kotton, an infectious disease expert who specializes in treating people with weak immune systems. “We’ve been in a sweet spot for maybe several months now as far as immunocompromised patients having good protection and then good treatment options,” said Kotton, a physician at Massachusetts General Hospital and a member of the Centers for Disease Control and Prevention’s independent vaccine advisory committee. But more immune evasive omicron subvariants such as BA.4.6, BA.2.75.2, BF.7, BQ.1 and BQ.1.1 are resistant to Evusheld, according to the National Institutes of Health. Scientists at Columbia University, for example, found Evusheld had completely lost its effectiveness against BA.4.6. And BQ.1 and BQ.1.1 are likely resistant to bebtelovimab, the monoclonal antibody developed by Eli Lily to prevent people with compromised immune systems who catch Covid from developing severe disease, according to NIH. That leaves people with compromised immune systems increasingly vulnerable as these subvariants increase in circulation in the U.S. As omicron BA.5 declines, this swarm of newer subvariants collectively make up about 38% of infections in the U.S., according to CDC data. Although Pfizer’s antiviral Paxlovid remains effective against the omicron subvariants, people who have had organ transplants often can’t take the pill because of the way it interacts with other drugs they need, Kotton said. “I’m concerned that the near future will be a challenging time for immunocompromised patients,” said Kotton. “The monoclonal antibodies in Evusheld are going to provide less protection and bebtelovimab is going to provide ineffective treatment for several of the emerging variants.” And help is not on the way at the moment. Kotton said she’s not aware of any monoclonal antibodies that are ready to replace the ones the subvariants are chipping away at. Jha acknowledged at the White House on Tuesday that the U.S. has dwindling treatment and prevention options for people with weak immune systems as Covid evolves. He blamed Congress for failing to pass $22.5 billion in funding for the nation’s Covid response due to Republican opposition. “We had hoped that over time as the pandemic went along, as our fight against this virus went along, we would be expanding our medicine cabinet,” Jha told reporters. “Because of lack of congressional funding that medicine cabinet has actually shrunk and that does put vulnerable people at risk.” Andrew Pekosz, a virologist at Johns Hopkins University, said finding ways to protect people with compromised immune systems is the most critical issue of the pandemic right now and it needs to be addressed quickly. “What we need to really work on is getting new antibody treatments out of the lab and into clinics,” Pekosz said. “In the lab, scientists know what next-generation monoclonal antibodies look like.” Kotton said people with compromised immune systems should stay up to date on their vaccines, which means getting the new booster that targets omicron BA.5. Those who have stayed up to date throughout the pandemic have received six shots by now. Those starting from scratch would receive a three-dose primary series of Moderna or Pfizer with the older generation shots and then a new booster that targets omicron, according to CDC guidelines. People with compromised immune systems should continue to exercise caution this winter, because the immune-resistant omicron subvariants could pick up in circulation as people gather for the holidays, Kotton said. But she noted that the group has been more diligent in wearing masks and practicing mitigation measures to avoid the virus than the rest of the population. The bigger problem is that the general population has largely moved on and is no longer taking basic precautions that could reduce transmissions and protect the vulnerable — such as wearing masks, Kotton said. “If we all were to mask more in public venues that would enhance the safety for them and allow them to have a higher likelihood of a safer return to many activities,” she said. Jha was asked by NBC News on Tuesday whether Biden telling people with weak immune systems to consult their physicians about precautions is an indication that the burden of responsibility has shifted to the individuals instead of the broader community. “As a society — as a caring society, we care about all Americans, particularly the most vulnerable Americans,” Jha said. “So it remains, I think, a collective responsibility for all of us to care about our fellow Americans who are immunocompromised.” The CDC recommends that people in communities where the Covid risk level is moderate to self test and wear a high-quality mask before meeting indoors with someone who is at high risk of getting sick. Those who are at high risk should wear a high-quality mask when indoors in public. When the Covid level is high, people in general should consider wearing high-quality masks and the vulnerable should consider avoiding indoor activities in public that aren’t essential, according to CDC. You can check your county’s Covid level at the CDC’s website.

|
Scooped by
Juan Lama
|
The B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has splintered into multiple subvariants with increased transmissibility and immune escape.1 At the time of this report, omicron subvariant BA.5 is the dominant global virus and has shown substantial immune escape as compared with previous omicron subvariants.2-5 BA.4.6 is a sublineage of BA.4 with two additional mutations in the spike protein (R346T and N658S) (Figure 1A) and has recently increased in prevalence in certain regions currently dominated by BA.5, including in the United States. The ability of BA.4.6 to evade neutralizing antibodies that were induced by infection or vaccination remains to be determined. We evaluated neutralizing antibody titers against five SARS-CoV-2 strains — WA1/2020 and omicron subvariants BA.1, BA.2, BA.4–BA.5, and BA.4.6 — in 19 participants who had been recently infected with the omicron BA.1 or BA.2 subvariant and in 16 participants who had been vaccinated and boosted with the original mRNA-1273 vaccine (Moderna) (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). In the cohort with previous omicron infection, all the participants except for one had been vaccinated; samples were obtained a median of 21 days after diagnosis of omicron infection. In this cohort, the median pseudovirus neutralizing antibody titer was 42,067 against WA1/2020, 6352 against BA.1, 3854 against BA.2, 1673 against BA.4–BA.5, and 630 against BA.4.6 (Figure 1B). The median neutralizing antibody titers against BA.4.6 were lower than the median titers against WA1/2020 by a factor of 67, against BA.1 by a factor of 10, against BA.2 by a factor of 6, and against BA.4–BA.5 by a factor of 2.7. In the mRNA-1273 vaccine cohort, participants were excluded if they had a known history of SARS-CoV-2 infection or positive results on nucleocapsid serologic analysis or if they had received immunosuppressive medications or other vaccines against SARS-CoV-2. Six months after the initial two mRNA-1273 immunizations, the median neutralizing antibody titer was 951 against WA1/2020, 28 against BA.2, 30 against BA.4–BA.5, and 23 against BA.4.6 (Figure 1C). At a median of 17 days after the first booster dose, the median neutralizing antibody titer was 16,011 against WA1/2020, 802 against BA.2, 449 against BA.4–BA.5, and 225 against BA.4.6. The median neutralizing antibody titer against BA.4.6 was lower than that against WA1/2020 by a factor of 71, against BA.2 by a factor of 4, and against BA.4–BA.5 by a factor of 2. Our data show that the BA.4.6 omicron subvariant markedly escaped neutralizing antibodies induced by infection or vaccination, with values that were lower than BA.5 titers by a factor of 2 to 2.7, which suggests continued evolution of SARS-CoV-2. These findings provide immunologic context for the increasing prevalence of BA.4.6 in populations in which BA.5 is currently dominant. Moreover, the R346T mutation had also recently been observed in other omicron subvariants, including BA.2.75 and BA.5, which suggests the biologic relevance of this mutation. The potential effect of the emergence of the BA.4.6 subvariant on vaccine boosters containing BA.5 immunogens or on infection with BA.5 remains to be determined. Published in NEJM (Oct. 19, 2022): https://doi.org/10.1056/NEJMc2212117

|
Scooped by
Juan Lama
|
Planas et al analyze the extent and duration of the neutralizing antibody response following vaccination with Pfizer BNT162b2 mRNA in the sera and nasal swabs from individuals with or without Omicron breakthrough infection, finding a short duration of neutralization against BA.5 after boosting and strong IgA response upon breakthrough infection. Background Since early 2022, Omicron BA.1 has been eclipsed by BA.2, which was in turn outcompeted by BA.5, that displays enhanced antibody escape properties. Methods Here, we evaluated the duration of the neutralizing antibody (Nab) response, up to 18 months after Pfizer BNT162b2 vaccination, in individuals with or without BA.1/BA.2 breakthrough infection. We measured neutralization of the ancestral D614G lineage, Delta and Omicron BA.1, BA.2, BA.5 variants in 300 sera and 35 nasal swabs from 27 individuals. Findings Upon vaccination, serum Nab titers were reduced by 10-, 15- and 25-fold for BA.1, BA.2 and BA.5, respectively, compared with D614G. We estimated that after boosting, the duration of neutralization was markedly shortened from 11.5 months with D614G to 5.5 months with BA.5. After breakthrough, we observed a sharp increase of Nabs against Omicron subvariants, followed by a plateau and a slow decline after 5-6 months. In nasal swabs, infection, but not vaccination, triggered a strong IgA response and a detectable Omicron neutralizing activity. Conclusions Thus, BA.5 spread is partly due to abbreviated vaccine efficacy, particularly in individuals who were not infected with previous Omicron variants. Published in Med (October 5, 2022)

|
Scooped by
Juan Lama
|
WASHINGTON — The Food and Drug Administration on Wednesday authorized the first redesign of coronavirus vaccines since they were rolled out in late 2020, setting up millions of Americans to receive new booster doses targeting Omicron subvariants as soon as next week. The agency cleared two options aimed at the BA.5 variant of Omicron that is now dominant: one made by Pfizer and its German partner BioNTech for use in people as young as 12, and the other by Moderna, for those 18 and older. The doses can be given at least two months since people last received a booster dose or completed their initial series of vaccinations. Biden administration officials have argued that even as researchers work to understand how protective the new shots might be, inoculating Americans again in the coming weeks could help curb the persistently high number of infections and deaths. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose,” Dr. Robert M. Califf, the F.D.A. commissioner, said in a statement on Wednesday. He added that the vaccine would “provide better protection against currently circulating variants.” An average of about 90,000 infections and 475 deaths are recorded every day around the United States, almost three years into a pandemic that has killed more than a million Americans and driven a historic drop in life expectancy. But there are also hopeful signs. Even with high case counts, fewer than 40,000 people are currently hospitalized with the virus, a decrease of 10 percent since early August and far fewer than during the Delta-driven surge last summer or the Omicron-fueled wave last winter. Deaths have also remained somewhat flat in recent weeks, a sign that vaccines are helping to prevent the worst outcomes of Covid-19. Ample evidence suggests that many Americans will hold back from getting the updated boosters, either because they are weary of the pandemic or may not feel urgency about an additional dose. With each new shot offered, there are fewer takers. As more companies bring workers back to offices and students return to campuses this fall, persuading Americans to get the updated booster shots will be a major challenge for the administration. The companies produced the retooled shots with extraordinary speed, a testament to the mRNA technology that Pfizer and Moderna have harnessed since the early months of the coronavirus outbreak. The Food and Drug Administration advised companies only two months ago on the formulation that they should adopt for the new vaccines. By later this week, millions of those doses will be delivered to states. The tight timeline meant that the companies went to federal regulators this summer with more limited data on the redesigned boosters than a traditional review process would call for, generating some controversy. Regulators authorized the vaccine without results from human trials, which have just started. Federal officials argue that because the coronavirus is evolving so quickly, human trials would be out of date before they can deliver results that could be used in the F.D.A.’s authorization decision. Instead, they are relying on the results of mouse trials and earlier human trials by Pfizer and Moderna of reformulations aimed at previous versions of the virus. The Biden administration is casting the shots as a standard upgrade that Americans should embrace ahead of potential surges in cases in the winter, like the flu shot, which is reconfigured every year to target more current versions of the influenza virus. The boosters are arriving during a period when the White House has been largely quiet on the pandemic. President Biden has rarely commented on the coronavirus in recent months, even after he tested positive in July. The White House no longer holds regular news briefings on the federal pandemic response, as it did in the first year of the administration — a reflection of the weariness of many Americans in keeping up with Covid precautions. “Covid-19 is the third leading cause of death in the United States. And it’s as if we’ve just accepted that that is going to be the case,” said Mercedes Carnethon, an epidemiologist at Northwestern University’s Feinberg School of Medicine. “I really hope as many people as possible will seek the updated booster so we can protect those who will have a terrible outcome.” Vaccinations remain the cornerstone of the federal government’s Covid strategy, even with tests and treatments widely available. The Biden administration has ordered over 170 million doses for the fall campaign, and officials do not expect shortages when they are rolled out. “If it’s freezing cold out and you have children, you’re going to dress them warmly. This is the concept here,” said Dr. Paul G. Auwaerter, the clinical director of the infectious diseases division at the Johns Hopkins University School of Medicine. “You’ll want to head into the respiratory season with a virus that we know has surprised us with a booster.” Exactly how protective the shots might be is unknown, Dr. Auwaerter said. He pointed to the modest increases in neutralizing antibodies that the companies found in vaccines they tested this year that targeted the original form of Omicron. How antibody levels would translate to protection with the new vaccines was unclear, he added. Experts warned against trying to choose Moderna’s shot over Pfizer’s or vice versa; with research in humans just beginning, scientists are months from knowing whether one brand offers better protection than the other. Many Americans have recently been infected with variants in the Omicron family and have some protection from their bouts with the virus, a development that federal agencies may take into account when recommending how the new shots are used. An advisory committee to the Centers for Disease Control and Prevention is scheduled to meet this week to make recommendations. “For most people, the risk of death is so low at this point, because they’ve gotten infected or vaccinated, or more likely both,” said Dr. Gregory A. Poland, a professor of medicine and infectious diseases and the director of the Vaccine Research Group at the Mayo Clinic. Dr. Poland, who has advised Moderna, Pfizer and White House officials on coronavirus vaccines, said updating booster shots the way the Food and Drug Administration did on Wednesday amounted to a “chase your tail” strategy, tweaking the design incrementally to try to keep up with a fast-changing virus. The new boosters, he said, could potentially save some lives among the elderly and those with immune deficiencies. But they were unlikely to make as substantial an impact with the rest of the population.

|
Scooped by
Juan Lama
|
SARS-CoV-2 omicron subvariants BA.1 and BA.2 became dominant in many countries in early 2022. These subvariants are now being displaced by BA.4 and BA.5. While natural infection with BA.1/BA.2 provides some protection against BA.4/BA.5 infection, the duration of this protection remains unknown. We used the national Portuguese COVID-19 registry to investigate the waning of protective immunity conferred by prior BA.1/BA.2 infection towards BA.5. We divided the individuals infected during the period of BA.1/BA.2 dominance (>90% of sample isolates) in successive 15-day intervals and determined the risk of subsequent infection with BA.5 over a fixed period. Compared with uninfected people, one previous infection conferred substantial protection against BA.5 re-infection at 3 months (RR=0.12; 95% CI: 0.11-0.12). However, although still significant, the protection was reduced by two-fold at 5 months post-infection (RR=0.24; 0.23-0.24). These results should be interpreted in the context of vaccine breakthrough infections, as the vaccination coverage in the individuals included in the analyses is >98% since the end of 2021. This waning of protection following BA.1/BA.2 infection highlights the need to assess the stability and durability of immune protection induced with the adapted vaccines (based on BA.1) over time. Preprint available at medRxiv (August 17, 2022): https://doi.org/10.1101/2022.08.16.22278820

|
Scooped by
Juan Lama
|
Following COVID-19 vaccine prime and boost, Lyke et al. find higher Omicron neutralization titers for homologous mRNA boost and heterologous mRNA and Ad26.COV2.S boost compared with homologous Ad26.COV2.S boost. Omicron titers rapidly decline by day 91 compared with prototypic D614G. Moderate differences in neutralization (<3-fold) were noted among Omicron sublineages. The Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exhibits reduced susceptibility to vaccine-induced neutralizing antibodies, requiring a boost to generate protective immunity. We assess the magnitude and short-term durability of neutralizing antibodies after homologous and heterologous boosting with mRNA and Ad26.COV2.S vaccines. All prime-boost combinations substantially increase the neutralization titers to Omicron, although the boosted titers decline rapidly within 2 months from the peak response compared with boosted titers against the prototypic D614G variant. Boosted Omicron neutralization titers are substantially higher for homologous mRNA vaccine boosting, and for heterologous mRNA and Ad26.COV2.S vaccine boosting, compared with homologous Ad26.COV2.S boosting. Homologous mRNA vaccine boosting generates nearly equivalent neutralizing activity against Omicron sublineages BA.1, BA.2, and BA.3 but modestly reduced neutralizing activity against BA.2.12.1 and BA.4/BA.5 compared with BA.1. These results have implications for boosting requirements to protect against Omicron and future variants of SARS-CoV-2. Published in Cell Reports Medicine (July 19, 2022): https://doi.org/10.1016/j.xcrm.2022.100679

|
Scooped by
Juan Lama
|
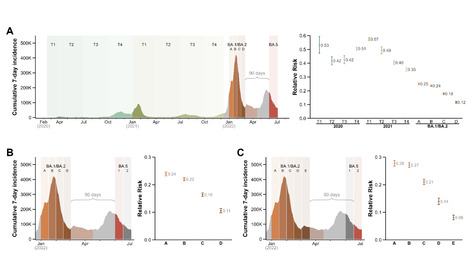
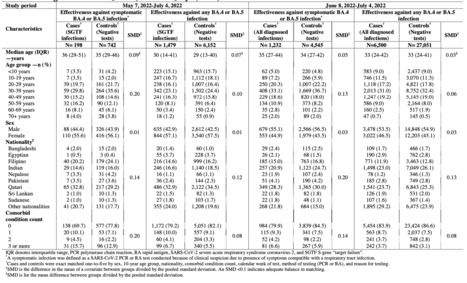
This study estimates the effectiveness of previous infection with SARSCoV2 in preventing reinfection with Omicron BA.4/BA.5 subvariants using a test negative, case control study design. Cases (SARSCoV2 positive test results) and controls (SARSCoV2 negative test results) were matched according to sex, 10 year age group, nationality, comorbid condition count, calendar week of testing, method of testing, and reason for testing. Effectiveness was estimated using the S gene target failure (SGTF) infections between May 7, 2022 and July 4, 2022. SGTF status provides a proxy for BA.4/BA.5 infections, considering the negligible incidence of other SGTF variants during the study. Effectiveness was also estimated using all diagnosed infections between June 8, 2022 and July 4, 2022, when BA.4/BA.5 dominated incidence. Effectiveness of a previous pre-Omicron infection against symptomatic BA.4/BA.5 reinfection was 15.1% (95% CI: -47.1 to 50.9%), and against any BA.4/BA.5 reinfection irrespective of symptoms was 28.3% (95% CI: 11.4 to 41.9%). Effectiveness of a previous Omicron infection against symptomatic BA.4/BA.5 reinfection was 76.1% (95% CI: 54.9 to 87.3%), and against any BA.4/BA.5 reinfection was 79.7% (95% CI: 74.3 to 83.9%). Results using all diagnosed infections when BA.4/BA.5 dominated incidence confirmed the same findings. Sensitivity analyses adjusting for vaccination status confirmed study results. Protection of a previous infection against BA.4/BA.5 reinfection was modest when the previous infection involved a preOmicron variant, but strong when the previous infection involved the Omicron BA.1 or BA.2 subvariants. Protection of a previous infection against BA.4/BA.5 was lower than that against BA.1/BA.2, consistent with BA.4/BA.5 greater capacity for immune system evasion than that of BA.1/BA.2. Preprint available at medRxiv (July 12, 2022): https://doi.org/10.1101/2022.07.11.22277448
|

|
Scooped by
Juan Lama
|
Background Older adults are at increased risk of SARS-CoV-2 Omicron infection and severe disease, especially those in congregate living settings, despite high SARS-CoV-2 vaccine coverage. It is unclear whether hybrid immunity (combined vaccination and infection) after one Omicron infection provides increased protection against subsequent Omicron reinfection in older adults. Methods Incidence of SARS-CoV-2 Omicron infection was examined in 750 vaccinated residents of long-term care and retirement homes in the observational cohort COVID in Long-Term Care Study in Ontario, Canada, within a 75-day period (July to September 2022). Risk of infection was assessed by Cox proportional hazards regression. Serum anti-spike and anti-RBD SARS-CoV-2 IgG and IgA antibodies, microneutralization titres, and spike-specific T cell memory responses, were examined in a subset of 318 residents within the preceding three months. Findings 133 of 750 participants (17.7%) had a PCR-confirmed Omicron infection during the observation period. Increased infection risk was associated with prior Omicron infection (at 9–29 days: 47.67 [23.73–95.76]), and this was not attributed to days since fourth vaccination (1.00 [1.00–1.01]) or residence outbreaks (>6 compared to ≤6: 0.95 [0.37–2.41]). Instead, reinfected participants had lower serum neutralizing antibodies to ancestral and Omicron BA.1 SARS-CoV-2, and lower anti-RBD IgG and IgA antibodies, after their initial Omicron infection. Interpretation Counterintuitively, SARS-CoV-2 Omicron infection was associated with increased risk of Omicron reinfection in residents of long-term care and retirement homes. Less robust humoral hybrid immune responses in older adults may contribute to risk of Omicron reinfection. Published in The Lancet eClinical Medicine (August 21, 2023):

|
Scooped by
Juan Lama
|
Rapid Antigen Tests (RAT) have become an invaluable tool for combating the COVID-19 pandemic. However, concerns have been raised regarding the ability of existing RATs to effectively detect emerging SARS-CoV-2 variants. We compared the performance of eight commercially available, emergency use authorized RATs against the Delta and Omicron SARS-CoV-2 variants using individual patient and serially diluted pooled clinical samples. The RATs exhibited lower sensitivity for Omicron samples when using PCR Cycle threshold (CT) value (a proxy for RNA concentration) as the comparator. Interestingly, however, they exhibited similar sensitivity for Omicron and Delta samples when using quantitative antigen concentration as the comparator. We further found that the Omicron samples had lower ratios of antigen to RNA, which offers a potential explanation for the apparent lower sensitivity of RATs for that variant when using CT value as a reference. Our findings underscore the complexity in assessing RAT performance against emerging variants and highlight the need for ongoing evaluation in the face of changing population immunity and virus evolution. Preprint available in medRxiv (Feb. 10, 2023): https://doi.org/10.1101/2023.02.09.23285583

|
Scooped by
Juan Lama
|
The geographic and evolutionary origins of the SARS-CoV-2 Omicron variant (BA.1), which was first detected mid-November 2021 in Southern Africa, remain unknown. We tested 13,097 COVID-19 patients sampled between mid-2021 to early 2022 from 22 African countries for BA.1 by real-time RT-PCR. By November-December 2021, BA.1 had replaced the Delta variant in all African sub-regions following a South-North gradient, with a peak Rt of 4.1. Polymerase chain reaction and near-full genome sequencing data revealed genetically diverse Omicron ancestors already existed across Africa by August 2021. Mutations, altering viral tropism, replication and immune escape, gradually accumulated in the spike gene. Omicron ancestors were therefore present in several African countries months before Omicron dominated transmission. These data also indicate that travel bans are ineffective in the face of undetected and widespread infection. Published in Science (Dec. 1, 2022): https://doi.org/10.1126/science.add8737

|
Scooped by
Juan Lama
|
Neutralizing antibodies that target the BA.4 and BA.5 subvariants were four-fold higher in people aged 55 and older who received the bivalent booster than in those who received a monovalent booster. New data from Pfizer and BioNTech on their bivalent Covid-19 vaccine suggests the updated product may be more protective against more recent Omicron subvariants than the original version of the vaccine, the companies said in a statement released Friday. The companies said the levels of neutralizing antibodies that target the BA.4 and BA.5 subvariants of the SARS-CoV-2 virus were four-fold higher in people aged 55 and older who received the bivalent booster than in similarly aged people who received a monovalent booster. The bivalent, which was given an emergency use authorization at the end of August, targets both the original version of the SARS-2 virus and the BA.4/BA.5 variants. Recently BA.5 has been the dominant strain in the United States, but an alphabet soup of newer subvariants — BA.4.6, BQ.1.1 among them — is starting to crowd it out. The new data from the companies only looks at what getting the the booster did to antibody levels in recipients. The trial did not test whether people who received the updated boosters were less likely to contract Covid than people who received one of the older boosters. “These data demonstrate that our BA.4/BA.5-adapted bivalent vaccine works as conceptually planned in providing stronger protection against the Omicron BA.4 and BA.5 sublineages,” Ugur Sahin, CEO and co-founder of BioNTech, said in the statement. “In the next step and as part of our science-based approach, we will continue to evaluate the cross-neutralization of the adapted vaccine against new variants and sublineages. Our aim is to provide broader immunity against Covid-19 caused by SARS-CoV-2, including Omicron and other circulating strains.” The companies also reported that one month after the trial participants got a dose of the bivalent booster, neutralizing antibodies targeting Omicron BA.4/BA.5 viruses increased 13.2-fold from pre-booster levels in adults who were older than 55 years of age; they increased 9.5-fold for adults 18 to 55 years of age. By comparison, in adults older than 55 who received a booster dose of the original vaccine, antibody titers to BA.4 and BA.5 rose 2.9-fold over the same period. Of late there have been a number of small studies that have tried to get an answer to the question of whether the updated vaccines are likely to be more protective than the original version against Omicron viruses. Three concluded that updating the vaccine did not make a difference while two suggested there was a benefit. But differences in the designs of the studies make them hard to compare to each other and to the Pfizer data. And at the end of the day, the important question is whether what was seen in terms of antibody production will translate into better protection for people who receive the bivalent vaccine, said Florian Krammer, a vaccinologist at Mount Sinai School of Medicine in Manhattan. He thought it might.
“A four-fold higher titer, that would be good,” said Krammer, who has done some paid consulting work for Pfizer. “Four-fold is usually the magical cut-off for a lot of us when we look at neutralization. Four-fold seems to mean something.” He cautioned though, that with different groups coming up with different estimates of whether the bivalent vaccine generated a significant improvement in antibody levels, “we really need to figure out what the truth is here and who is right in terms of measuring.” Eric Topol, director of the Scripps Research Translational Institute, thought the results were promising. “I think this is encouraging,” Topol told STAT. “We just need more people to get the darn booster.” Data from the Centers for Disease Control show uptake of the bivalent boosters has been slow, with only 26.3 million people having received it so far. Even in the highest risk age group, people 65 and older, uptake has been modest. To date only 23% of Americans in that age group have received a bivalent booster. Pfizer press release (Nov. 04, 2022) available here: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-updated-clinical-data-omicron

|
Scooped by
Juan Lama
|
Monoclonal antibodies (mAbs) offer a treatment option for individuals with severe COVID-19 and are especially important in high-risk individuals where vaccination is not an option. Given the importance of understanding the evolution of resistance to mAbs by SARS-CoV-2, we reviewed the available in vitro neutralization data for mAbs against live variants and viral constructs containing spike mutations of interest. Unfortunately, evasion of mAb-induced protection is being reported with new SARS-CoV-2 variants. The magnitude of neutralization reduction varied greatly among mAb–variant pairs. For example, sotrovimab retained its neutralization capacity against Omicron BA.1 but showed reduced efficacy against BA.2, BA.4 and BA.5, and BA.2.12.1. At present, only bebtelovimab has been reported to retain its efficacy against all SARS-CoV-2 variants considered here. Resistance to mAb neutralization was dominated by the action of epitope single amino acid substitutions in the spike protein. Although not all observed epitope mutations result in increased mAb evasion, amino acid substitutions at non-epitope positions and combinations of mutations also contribute to evasion of neutralization. This Review highlights the implications for the rational design of viral genomic surveillance and factors to consider for the development of novel mAb therapies. In this Review, Carabelli, Robertson and colleagues explore data on the neutralization of globally circulating variants of concern by monoclonal antibodies (mAbs) and discuss how knowledge of the dynamics of viral evasion of mAbs can contribute to viral surveillance and the development of novel mAb treatments, as well as inform predictions of resistance that may arise in the future. Published in Nature (October 28, 2022): https://doi.org/10.1038/s41579-022-00809-7

|
Scooped by
Juan Lama
|
Top SARS-CoV-2 variants with increased resistance to vaccine and therapeutic antibodies. Seven new lineages including BA.2.75, BA.2.75.2, BA.4.6, BF.7, BQ.1, BQ.1.1 and XBB.1 are circulating globally, with different sub-variants growing faster in different regions. Global and US frequencies of the eight variants as of October 29, 2022 are shown. In the US, the combined pool of new variants already represents 50% of the viral genomes sequenced last week. Top graph shows the spike mutations for each lineages appearing in at least 50% of the viral genomes sequenced for each variant. Key mutations in spike conferring escape from antibodies (vaccine or therapeutics) include R346T, K444T, L452R, N460K, and F486S/V. Data from GISAID, US CDC, cov-spectrum.org, and outbreak.info used to generate the charts at https://lnkd.in/g7YYq4y

|
Scooped by
Juan Lama
|
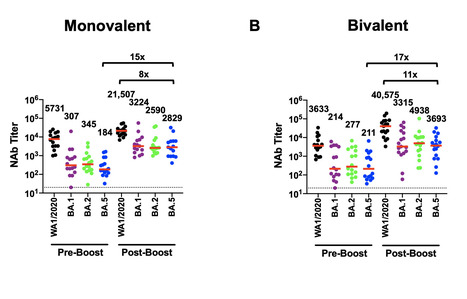
Waning immunity following mRNA vaccination and the emergence of SARS-CoV-2 variants has led to reduced mRNA vaccine efficacy against both symptomatic infection and severe disease. Bivalent mRNA boosters expressing the Omicron BA.5 and ancestral WA1/2020 Spike proteins have been developed and approved, because BA.5 is currently the dominant SARS-CoV-2 variant and substantially evades neutralizing antibodies (NAbs). Our data show that BA.5 NAb titers were comparable following monovalent and bivalent mRNA boosters. Preprint available in bioRxiv: https://doi.org/10.1101/2022.10.24.513619

|
Scooped by
Juan Lama
|
Significance Tracking the animal reservoir of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants is important for understanding the current COVID-19 pandemic and preventing future pandemics. Speculations about the source of the omicron variant are abundant, yet experimental evidence has been scarce. Here, we provide the structural information on how omicron recognizes its mouse receptor. Our study demonstrates that the omicron mutations in the receptor-binding region are structurally adapted to mouse angiotensin-converting enzyme 2 (ACE2), informing an understanding of the origin of the omicron variant and the evolution of SARS-CoV-2. It may facilitate epidemiological surveillance of SARS-CoV-2 in animals to prevent future coronavirus pandemics. Abstract The sudden emergence and rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron variant has raised questions about its animal reservoir. Here, we investigated receptor recognition of the omicron’s receptor-binding domain (RBD), focusing on four of its mutations (Q493R, Q498R, N501Y, and Y505H) surrounding two mutational hotspots. These mutations have variable effects on the RBD’s affinity for human angiotensin-converting enzyme 2 (ACE2), but they all enhance the RBD’s affinity for mouse ACE2. We further determined the crystal structure of omicron RBD complexed with mouse ACE2. The structure showed that all four mutations are viral adaptations to mouse ACE2: three of them (Q493R, Q498R, and Y505H) are uniquely adapted to mouse ACE2, whereas the other one (N501Y) is adapted to both human ACE2 and mouse ACE2. These data reveal that the omicron RBD was well adapted to mouse ACE2 before omicron started to infect humans, providing insight into the potential evolutionary origin of the omicron variant. Published in PNAS (Oct.18, 2022):

|
Scooped by
Juan Lama
|
BACKGROUND The safety and immunogenicity of the bivalent omicron-containing mRNA-1273.214 booster vaccine are not known. METHODS In this ongoing, phase 2–3 study, we compared the 50-μg bivalent vaccine mRNA-1273.214 (25 μg each of ancestral Wuhan-Hu-1 and omicron B.1.1.529 [BA.1] spike messenger RNAs) with the previously authorized 50-μg mRNA-1273 booster. We administered mRNA-1273.214 or mRNA-1273 as a second booster in adults who had previously received a two-dose (100-μg) primary series and first booster (50-μg) dose of mRNA-1273 (≥3 months earlier). The primary objectives were to assess the safety, reactogenicity, and immunogenicity of mRNA-1273.214 at 28 days after the booster dose. RESULTS Interim results are presented. Sequential groups of participants received 50 μg of mRNA-1273.214 (437 participants) or mRNA-1273 (377 participants) as a second booster dose. The median time between the first and second boosters was similar for mRNA-1273.214 (136 days) and mRNA-1273 (134 days). In participants with no previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the geometric mean titers of neutralizing antibodies against the omicron BA.1 variant were 2372.4 (95% confidence interval [CI], 2070.6 to 2718.2) after receipt of the mRNA-1273.214 booster and 1473.5 (95% CI, 1270.8 to 1708.4) after receipt of the mRNA-1273 booster. In addition, 50-μg mRNA-1273.214 and 50-μg mRNA-1273 elicited geometric mean titers of 727.4 (95% CI, 632.8 to 836.1) and 492.1 (95% CI, 431.1 to 561.9), respectively, against omicron BA.4 and BA.5 (BA.4/5), and the mRNA-1273.214 booster also elicited higher binding antibody responses against multiple other variants (alpha, beta, gamma, and delta) than the mRNA-1273 booster. Safety and reactogenicity were similar with the two booster vaccines. Vaccine effectiveness was not assessed in this study; in an exploratory analysis, SARS-CoV-2 infection occurred in 11 participants after the mRNA-1273.214 booster and in 9 participants after the mRNA-1273 booster. CONCLUSIONS The bivalent omicron-containing vaccine mRNA-1273.214 elicited neutralizing antibody responses against omicron that were superior to those with mRNA-1273, without evident safety concerns. (Funded by Moderna; ClinicalTrials.gov number, NCT04927065. opens in new tab.) Published in NEJM (Sept.16, 2022): https://doi.org/10.1056/NEJMoa2208343

|
Scooped by
Juan Lama
|
Consecutive waves of SARS-CoV-2 infection have been driven in part by the repeated emergence of variants with mutations that confer resistance to neutralizing antibodies Nevertheless, prolonged or repeated antigen exposure generates diverse memory B-cells that can produce affinity matured receptor binding domain (RBD)-specific antibodies that likely contribute to ongoing protection against severe disease. To determine how SARS-CoV-2 omicron variants might escape these broadly neutralizing antibodies, we subjected chimeric viruses encoding spike proteins from ancestral, BA.1 or BA.2 variants to selection pressure by a collection of 40 broadly neutralizing antibodies from individuals with various SARS-CoV-2 antigen exposures. Notably, pre-existing substitutions in the BA.1 and BA.2 spikes facilitated acquisition of resistance to many broadly neutralizing antibodies. Specifically, selection experiments identified numerous RBD substitutions that did not confer resistance to broadly neutralizing antibodies in the context of the ancestral Wuhan-Hu-1 spike sequence, but did so in the context of BA.1 and BA.2. A subset of these substitutions corresponds to those that have appeared in several BA.2 daughter lineages that have recently emerged, such as BA.5. By including as few as 2 or 3 of these additional changes in the context of BA.5, we generated spike proteins that were resistant to nearly all of the 40 broadly neutralizing antibodies and were poorly neutralized by plasma from most individuals. The emergence of omicron variants has therefore not only allowed SARS-CoV-2 escape from previously elicited neutralizing antibodies but also lowered the genetic barrier to the acquisition of resistance to the subset of antibodies that remained effective against early omicron variants. Preprint available in bioRxiv (August 19, 2022): https://doi.org/10.1101/2022.08.17.504313

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration is using a controversial strategy to evaluate the next generation of COVID-19 boosters. The approach is stirring debate as the agency works to make new, hopefully improved, boosters available in September to help prevent severe disease and save lives in the fall and winter. For the first time, the FDA is planning to base its decision about whether to authorize new boosters on studies involving mice instead of humans. "For the FDA to rely on mouse data is just bizarre, in my opinion," says John Moore, an immunologist at Weill Cornell Medicine in New York. "Mouse data are not going to be predictive in any way of what you would see in humans." But others defend the approach, arguing that the country has had enough experience with the vaccines at this point to be confident the shots are safe and that there's not enough time to wait for data from human studies. "We have 500 people a day dying of coronavirus right now. Those numbers sadly might very well rise in the fall and the winter. The question is: 'Can we do something better?'" says Dr. Ofer Levy, a pediatrics and infectious disease researcher at Harvard Medical School who also advises the FDA. "And I think the answer is: 'We can, by implementing this approach.'" The U.K. just approved a new booster The United Kingdom just approved a new booster that targets both the original strain of the virus and the original omicron variant, called BA.1 — a so-called bivalent vaccine. But the FDA rejected BA.1 bivalent boosters last spring. Instead, the FDA told the vaccine companies that make the mRNA vaccines, Moderna and Pfizer and BioNTech, to develop bivalent vaccines that target the dominant omicron subvariants — BA.4 and BA.5 — in the hopes they will offer stronger, longer-lasting protection. That's why the FDA decided to use a new, streamlined strategy for testing the new boosters. The agency is asking the companies to initially submit only the results of tests on mice. Regulators will rely on those results, along with the human neutralizing antibody data from the BA.1 bivalent booster studies, to decide whether to authorize the boosters. The companies will continue to gather more data from human studies; those results probably won't be available until late October or early November. But the big concern is the boosters may not work as well as the mouse data might suggest. Mouse experiments are notoriously unreliable. And with the government telling people not to get the old boosters now and rejecting the first bivalent vaccines, the FDA really needs good evidence that the BA.4/5 boosters are in fact better, critics say. "We need to make sure that we have solid immunogenicity data in people to show that you have a dramatically greater neutralizing antibody response against BA.4, BA.5," says Dr. Paul Offit of the University of Pennsylvania, who also advises the FDA. "I think anything short of that is not acceptable." Some also worry that the approach may further erode the long-faltering efforts to persuade people to get boosted. "I think it would be good to have neutralizing antibody data in a small group of humans," says Dr. Monica Gandhi, an infectious disease researcher at the University of California, San Francisco. "Otherwise, extrapolation may be considered too great." But others agree the time constraints mean the country can't wait for more evidence. The billions of people who have gotten Moderna and Pfizer-BioNTech mRNA vaccines show how safe they are, those experts say. The new booster will be identical to the original vaccines except it will contain genetic coding for two versions of the protein the virus uses to infect cells — the protein from the original vaccine and proteins from the BA.4 and BA.5 omicron subvariants. And some scientists say health officials know enough about how vaccines work to start handling the COVID-19 vaccines like the flu vaccines, which are changed every year to try to match whatever strains are likely to be circulating but aren't routinely tested again every year. "We're going to use all of these data that we've learned through not only from this vaccine but decades of viral immunology to say: 'The way to be nimble is that we're going to do those animal studies," says Deepta Bhattacharya, an immunobiologist at the University of Arizona College of Medicine in Tucson. "We're really not going out too far on a limb here." The companies are expected to submit their data to the FDA by the end of the month and the administration hopes to make millions of doses of the new boosters available starting in September.

|
Scooped by
Juan Lama
|
Since the end of 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant outcompeted other variants and took over the world. After the emergence of original Omicron BA.1, Omicron BA.2 subvariant emerged and outcompeted BA.1. As of July 2022, some BA.2 subvariants, including BA.2.12.1, BA.4 and BA.5, emerged in multiple countries and begun outcompeting original BA.2. Moreover, a novel BA.2 subvariant, BA.2.75, was detected in eight countries including India at the end of June 2022, and preliminary investigations suggest that BA.2.75 is more transmissible over the other BA.2 subvariants. On July 7, 2022, the WHO classified BA.2.75 as a variant-of-concern lineage under monitoring. We have recently demonstrated that BA.4/5 is highly resistant to a therapeutic monoclonal antibody, cilgavimab, than BA.2. The resistance of SARS-CoV-2 variants to therapeutic antibodies can be attributed to the mutations in the viral spike protein. Compared to the BA.2 spike, BA.2.12.1 and BA.4/5 respectively bear two and four mutations in their spike proteins. On the other hand, the majority of BA.2.75 spike bears nine substitutions. The fact that the mutation number in the BA.2.75 spike is larger than those in the BA.4/5 spike raises the possibility that the BA.2.75 spike significantly reduces sensitivity towards therapeutic monoclonal antibodies than BA.2 and BA.4/5. In this study, we generated pseudoviruses harboring the spike proteins of BA.2.75, BA.4/5 and BA.2 and evaluated the efficacy of ten therapeutic monoclonal antibodies and three antibody cocktails against BA.2.75. Preprint available in medRxiv (July 15, 2022): https://doi.org/10.1101/2022.07.14.500041
|



 Your new post is loading...
Your new post is loading...