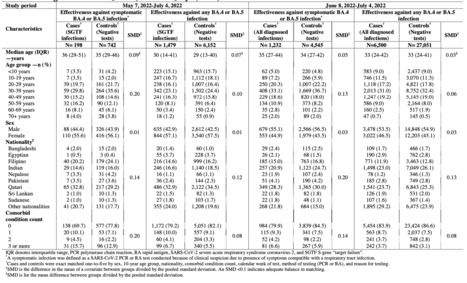
Data from Qatar provide strongest evidence yet that COVID-19 vaccines can stop strains thought to pose a threat to immunization efforts. Qatar’s second wave of COVID-19 was a double whammy. In January, after months of relatively few cases and deaths, the Gulf nation saw a surge driven by the fast-spreading B.1.1.7 variant, which was first identified in the United Kingdom. Weeks later, the B.1.351 strain, which is linked to reinfections and dampened vaccine effectiveness, took hold. Amid this storm, researchers in Qatar have found some of the strongest evidence yet that current vaccines can quell variants such as B.1.351. Clinical trials in South Africa — where B.1.351 was first identified — had suggested that vaccines would take a hit against such variants. But this study offers a fuller picture of what countries battling such variants can expect. People in Qatar who received two doses of the Pfizer–BioNTech vaccine were 75% less likely to develop a case of COVID-19 caused by B.1.351 than were unvaccinated people, and had near-total protection from severe disease caused by that strain. The findings — published on 5 May in The New England Journal of Medicine1 — suggest that current RNA vaccines are a potent weapon against the most worrisome immune-evading variants. Pfizer, based in New York City, and BioNTech, in Mainz, Germany, are developing an updated RNA vaccine targeting B.1.351, as is Moderna, based in Cambridge, Massachusetts. Early results from Moderna’s efforts suggest that a booster shot of the updated vaccine triggers a strong response against B.1.351. “I think this variant is probably the worst of all the variants we know,” says Laith Jamal Abu-Raddad, an infectious-disease epidemiologist at Weill Cornell Medicine—Qatar in Doha, who led the Qatari study. “We have the tools, despite these variants, to control at least the severe forms of infection — and this should work quite well on transmission.”
Weaker protection
Researchers in South Africa identified B.1.351 in late 2020, and it’s now the predominant strain there. Laboratory studies show that the variant harbours mutations that blunt the effects of virus-blocking antibodies, and trials suggest that some COVID-19 vaccines are significantly less effective against the strain than against others. Early lab research suggested that RNA vaccines, including the Pfizer–BioNTech jab, would be weakened by B.1.351, but probably not fully compromised. In April, the companies announced that a small trial in South Africa had found the vaccine to be fully effective against B.1.351, but the study of 800 people recorded a total of just 6 infections caused by B.1.351 in the placebo group, so efficacy might have been much lower. Abu-Raddad’s team analysed tens of thousands of COVID-19 cases that occurred between the start of Qatar’s vaccination campaign in late December and the end of March. Genome sequencing showed that B.1.1.7 and B.1.351 were the predominant coronavirus lineages during this period and, from mid-February, each accounted for about half of the country’s cases. The researchers compared SARS-CoV-2 infection rates in vaccinated people with those in unvaccinated controls. People who received two vaccine doses were about 90% less likely to develop an infection caused by B.1.1.7, echoing findings from Israel, the United Kingdom and elsewhere. The researchers identified around 1,500 ‘breakthrough’ infections caused by the B.1.351 variant in vaccinated individuals, but only 179 of these occurred more than 2 weeks after the second dose. There were hardly any severe cases of COVID-19 caused by either B.1.1.7 or B.1.351 among fully vaccinated individuals. “Even though there were breakthrough infections, they didn’t lead to hospitalization and death, except very, very rarely,” says Abu-Raddad. Two people died of COVID-19 caused by B.1.351 after receiving their second vaccine dose, but it is very likely that they were infected before the protective effects of the booster shot began. “If, a year ago, I told somebody we would have 75% effectiveness against the worst variants we had, they would consider this extremely good news,” Abu-Raddad adds.
Promising data
Shabir Madhi, a vaccinologist at the University of the Witwatersrand in Johannesburg, South Africa, says the Qatari results are promising. The comparatively high levels of virus-blocking antibodies triggered by two doses of an RNA vaccine probably explain why it confers better protection against B.1.351 than do other vaccines, such as the one developed by the University of Oxford, UK, and pharmaceutical company AstraZeneca in Cambridge, UK. But Madhi expects that other vaccines will also prevent severe disease caused by that variant. In another 5 May New England Journal of Medicine study2, his team reported that the jab produced by biotechnology company Novavax in Gaithersburg, Maryland, lowered the risk of getting COVID-19 by 60% in participants without HIV in a South African trial involving more than 6,000 people. As-yet unpublished data show that the vaccine was highly effective against severe cases of COVID-19 caused by B.1.351, with no cases in vaccinated individuals and five in the placebo arm. If vaccine efficacy is lower against B.1.351, even highly successful immunization programmes in countries affected by the variant might not reduce cases to the same extent as in countries dealing with less troublesome strains, says Madhi. “Nevertheless, by protecting high-risk individuals, we could still return to a relatively normal lifestyle, even with ongoing circulation.” Qatar, where more than one-third of the population has received at least one dose of the vaccine, might provide an early glimpse at how the worst coronavirus variants can be controlled. Abu-Raddad says there is evidence that the Pfizer–BioNTech vaccine might also be highly effective at blocking transmission of B.1.351. And after cases of the variant peaked in mid-April, he says, “things have been going extremely well, the numbers are going down very, very rapidly”.
Publication cited available in NEJM (May 5, 2021):
https://www.nejm.org/doi/10.1056/NEJMc2104974
See also The Lancet (May 5, 2021):
https://doi.org/10.1016/S0140-6736(21)00947-8



 Your new post is loading...
Your new post is loading...