 Your new post is loading...

|
Scooped by
Juan Lama
|
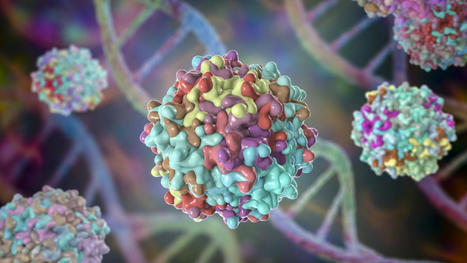
A research team led by gene editing pioneer David R. Liu, PhD, reports the application of base editing technology to develop a one-time treatment for spinal muscular atrophy (SMA), showing promising effectiveness in cell and mouse models. Liu, a professor at Harvard University, the Broad Institute, and an investigator with the Howard Hughes Medical Institute, together with 16 co-authors, published their findings today in Science, in a report entitled, “Base editing rescue of spinal muscular atrophy in cells and in mice.” The paper is the first one published in a peer-reviewed journal that details a base editing approach to treating SMA—the leading genetic cause of infant mortality. Two months ago, Benjamin P. Kleinstiver, PhD, assistant professor in the Center for Genomic Medicine at Massachusetts General Hospital (MGH) and Harvard Medical School, and 12 co-authors posted a related preprint on bioRxiv detailing successful results from their study to optimize and implement a base editing treatment for SMA. In SMA, patients have either a loss of, or mutations in, the SMN1 gene. The result is a lack of its corresponding protein SMN, which is critical to cellular functions that include those required for embryo development. Liu and co-authors detailed how they restored SMN protein abundance to normal levels and rescued disease phenotypes in cell and mouse models of SMA by developing one-time genome editing approaches targeting another gene, called SMN2. SMN2 differs from SMN1 at just one position—a single C-to-T mutation in exon 7. The result is a defective truncated SMN protein that fails to fully compensate for the loss of SMN1, but which provides just enough SMN protein activity to allow babies to be born with SMA. In the most common SMA cases (type 1), those babies typically live less than two years without treatment. Liu and colleagues developed a customized base editor (out of more than 40 tested) that directly converts SMN2 to the same sequence as SMN1 by reversing the C-to-T mutation that occurred during the duplication of SMN1 to SMN2. The investigators reported that their strategy converted SMN2 to SMN1 with near-ideal editing efficiency of 99% in cells, fully restoring SMN protein levels ~40-fold to normal levels, while generating minimal off-target edits. “Because all SMA patients have SMN2—otherwise loss of SMN1 would not allow them to be born—this base editing approach to treating SMA also should be applicable to all SMA patients, regardless of the specific mutation that caused their SMN1 loss,” Liu said.

|
Scooped by
Juan Lama
|
The animals lived longer and showed milder symptoms than untreated mice, although they didn't survive as long as wild type mice. Earlier this year, the US Food and Drug Administration approved the most expensive drug ever to hit the market, a gene therapy for spinal muscular atrophy. SMA is a neuromuscular disorder that, in severe cases, can lead to infant death. The genetic correction is currently used to treat affected newborns, but as symptoms for some types of SMA may appear before birth, an earlier treatment would be potentially more effective. In a study published December 4 in Molecular Therapy, researchers were able to fix a mutation in the survival motor neuron 1 (SMN1) gene—which causes SMA in humans—in mice modelling the disease, while they were still inside their mothers’ uterus. The treated mice lived longer and had fewer symptoms than untreated animals. Tippi MacKenzie, a fetal and pediatric surgeon at the University of California, San Francisco, who did not participate in this study, says it is an important paper because it is the first time fetal gene therapy has succeeded in SMA mice. “Before you even think about doing something in patients, you have to first do it in the disease model of the mouse . . . so this group has supplied a very important piece to the literature,” she adds. SMN1 encodes an essential protein for the maintenance of motor neurons, which are nerve cells in the brain and spinal cord responsible for controlling muscle movement. The result in children with mutations in the gene is the loss of motor neurons, leading to muscle weakness and associated complications. SMA affects one out of every 6,000 to 10,000 babies. Correcting the SMN1 sequence is a potentially efficient treatment for those born with SMA. Zolgensma, the recently approved medication for this disorder, consists of an intravenous administration of an adeno-associated virus that ferries a functional copy of the SMN1 gene to the brain. To see if the same fix could be accomplished before birth, the research team tested two different injection methods: one into the placenta (intraplacental or IP) and the other into one of the brain lateral ventricles (intracerebroventricular or ICV). The latter proved to be more effective. By injecting the viral vector into the fetus’s brain, the virus will go directly into the cerebrospinal fluid, “and it will transduce motor neurons in the spinal cord with a very high efficiency, compared to the IP [injection],” says Afrooz Rashnonejad. who participated in this study while working at Ege University in Izmir, Turkey, but has recently moved to Nationwide Children’s Hospital in Columbus, Ohio. Rashnonejad and her colleagues then monitored the injected mice that were carried to term. Those treated with the vector carrying a functional copy of SMN1 lived a median lifespan of 63 or 105 days (depending on the type of cassette carrying the gene), much longer than untreated SMA mice, which did not survive more than 14 days, but still less than wildtype pups, which had a median lifespan of 405 days. The treated mice were also heavier than untreated mice, but smaller than healthy mice.... Published in Molecular Therapy (August 31, 2019): https://doi.org/10.1016/j.ymthe.2019.08.017

|
Scooped by
Juan Lama
|
Zolgensma, a lifesaving treatment for infants and the world’s most expensive drug, has been used to treat 100 patients since its launch and brought in $160 million for its maker Novartis (NVS) last quarter, beating analysts’ expectations. The gene therapy is a treatment for spinal muscular atrophy, a rare and devastating neurological disease. It carries a record price tag of $2.1 million, or an annualized cost of $425,000 per year for five years. Novartis said Tuesday that roughly 99% of SMA patients who qualified for Zolgensma got coverage of the one-time therapy, although some had to go through an approval process to receive the drug. The company also indicated that it made progress in striking deals with health plans to cover the drug, saying that agreements are in place covering roughly 90% of patients insured commercially and roughly 30% of patients covered by Medicaid. “I think the overall uptake that we’re seeing very early in the launch speaks to the promise of the product, the physician excitement, and the parent and patient desire to get kids on gene therapy,” said Dave Lennon, president of AveXis, Novartis’s gene therapy business. The strong sales numbers came over a summer that saw a controversy involving manipulation of data used to support Zolgensma’s approval by the Food and Drug Administration. In an unusual rebuke, the agency said in August that AveXis knew that preclinical data had been falsified before the drug was approved in May, but did not inform the Food and Drug Administration until later. The agency said that the drug should stay on the market, but the scandal sparked anger from lawmakers and a pledge from Novartis’s CEO, Vas Narasimhan, to move more quickly on disclosing issues around data integrity. Lennon said Novartis worked quickly to reassure patients and physicians of Zolgensma’s quality, and the data manipulation scandal had “no real impact” on the treatment’s commercial performance, Lennon said. Zolgensma’s early success could be a worrying sign for Biogen (BIIB), whose Spinraza has been the treatment of choice for SMA since its approval in 2016. More than 50% of patients treated with Zolgensma had switched over from Spinraza, according to Novartis, preferring a one-time therapy over Biogen’s every-four-months treatment. Zolgensma’s sales outstripped what analysts had forecasted. One average of analysts’ projections, from Bloomberg, had pegged the drug’s sales for the quarter at $86 million. Another compiler of financial estimates, FOA, had gauged analysts’ consensus at $106 million. Novartis expects revenue to grow. The company is counting on approvals for Zolgensma in Europe and Japan next year, and it recently presented data demonstrating the gene therapy’s effects on older SMA patients. Novartis is also counting on an expansion of newborn testing for SMA, which is currently done in 30% of U.S. states but could rise to 70% by 2020, according to the company. “Time is neurons for these kids, and we really want to make sure they get gene therapy as soon as possible,” Lennon said.
|

|
Scooped by
Juan Lama
|
Long-term follow-up study data for Novartis’ one-time gene therapy for spinal muscular atrophy has shown promising milestone achievements. Data from LT-001, Novartis’ ongoing 15-year long-term follow-up (LTFU) study of one-time gene therapy Zolgensma® (onasemnogene abeparvovec), revealed significant milestone achievements for spinal muscular atrophy (SMA) patients. The latest data from two LTFU studies LT-001 and LT-002 was presented at the 2023 MDA Clinical & Scientific Conference. Highlights included: - Sustained durability up to 7.5 years post-dosing; 100 percent achievement of all assessed milestones in children treated prior to SMA symptom onset
- Children in LT-001 treated after SMA symptom onset maintained or achieved additional milestones up to 7.5 years post one-time intravenous (IV) infusion
- All children (100 percent) in the presymptomatic intravenous cohort of LT-002 maintained or achieved all assessed motor milestones, including independent walking
- Additionally, children with SMA Type 2 treated with investigational intrathecal OAV101 maintained or achieved new development gains.
“…the fact that we’re seeing [some patients from the long-term follow up studies] maintain and, in some cases, gain motor milestones when they are nearly eight years old is truly transformational,” shared Dr Jerry R Mendell of Nationwide Children’s Hospital. Additional data Interim results from the 15-year LT-002 study was also presented. All patients (100 percent) were shown to maintain motor milestones achieved during their respective parent studies in the follow-up period. Results from the IV-administered cohort, which included 63 patients, demonstrated a single administration of Zolgensma provided consistent, substantial and durable efficacy over time. Notably, in the presymptomatic IV cohort, all children (100 percent) either maintained the highest milestone achieved during the parent study (walking alone) or achieved the milestone by the data-cut off. In total, six patients treated prior to SMA symptom onset and 16 treated after SMA symptom onset achieved new motor milestones in the follow-up period. There were no deaths, no serious treatment-emergent adverse events (TEAEs) related the gene therapy and no serious TEAEs that resulted in study discontinuation. The most frequently reported events were acute respiratory failure, dehydration, and pneumonia (each in five patients, 38.5 percent). No new safety signals were identified. Additionally, findings from the ongoing RESTORE registry were presented at the MDA conference. Patients with four or more copies of the survival motor neuron 2 (SMN2) gene treated with Zolgensma alone attained improvements in survival, motor function and achieved new milestones. The registry provides real-world evidence data to enhance the understanding of SMA patients cared for in routine practice. These results continue to highlight the importance of early identification and intervention to optimise outcomes for all SMA patients. “Data from the LT-001 and LT-002 studies showed that, regardless of the patient’s symptomatic status at the time of treatment, Zolgensma IV is an effective and durable treatment option,” commented Dr Sitra Tauscher-Wisniewski, Vice President Clinical Development & Analytics, Novartis Gene Therapies. NICE’s recommendation of gene therapy Zolgensma On 16 March 2023, the National Institute for Health and Care Excellence (NICE) recommended Zolgensma as an option in babies with presymptomatic 5q spinal muscular atrophy (SMA) with a bi-allelic mutation in the SMN1 gene and up to three copies of the SMN2 gene. This recommendation will allow routine access to onasemnogene abeparvovec, designed to help halt disease progression before symptom onset. Imran Kausar, General Manager at Novartis Gene Therapies UK commented on NICE’s decision: “Zolgensma will be the first treatment to be routinely commissioned for presymptomatic babies in England, and as it is imperative to diagnose SMA and begin treatment as early as possible, we welcome the decision by NICE for this recommendation.” There is no national newborn screening programme for SMA in the UK. Infants without a family history are only diagnosed if symptoms are identified. As this can take up to six months, an established programme is important. Following decision by the NICE committee that onasemnogene abeparvovec is effective in treating presymptomatic SMA, final guidance is expected to be published on 19 April 2023. Novartis Press Release (March 20, 2023) available here: https://www.novartis.com/news/media-releases/novartis-shares-zolgensma-long-term-data-demonstrating-sustained-durability-75-years-post-dosing-100-achievement-all-assessed-milestones-children-treated-prior-sma-symptom-onset

|
Scooped by
Juan Lama
|
The FDA has imposed a partial hold on clinical trials for intrathecal administration of the Novartis gene therapy AVXS-101, which won the FDA's first approval for treating some forms of spinal muscular atrophy (SMA) in May under the name Zolgensma® (onasemnogene abeparvovec-xioi). The partial hold does not affect the marketing of Zolgensma or clinical trials assessing intravenous (IV) delivery of AVXS-101, Novartis emphasized. However, the hold affects studies assessing AVXS-101 administered as an injection into the spinal canal in patients with SMA Type 2. As a result of the hold, enrollment in the high dose cohort has been stopped in the Phase I STRONG trial (NCT03381729), an ongoing, open-label, dose-comparison, multi-center trial designed to evaluate the efficacy, safety, and tolerability of one-time intrathecal administration of AVXS-101. The low- and mid-dose cohort enrollment has previously been completed and interim results have been presented. The pharma giant said the partial hold followed AveXis, A Novartis Company, alerting authorities and clinical trial investigators about animal findings from a small, AveXis-launched preclinical study showing dorsal root ganglia (DRG) mononuclear cell inflammation, sometimes accompanied by neuronal cell body degeneration or loss. “The clinical significance of the DRG inflammation observed in this preclinical animal study is not known and was not seen in prior animal studies with AVXS-101,” Novartis said in a statement, adding that DRG inflammation can be associated with sensory effects. “We have completed a thorough review of human safety data from all available sources to date and no adverse effects related to sensory changes have been seen in AVXS-101 intrathecal or Zolgensma. We are working with health authorities to confirm further guidance to clinical investigators.” Novartis also said it will continue to closely monitor for any reports of related safety events in patients, adding: “We remain confident that the overall benefit-risk profile for patients on treatment is favorable.” The partial clinical hold comes a month after another safety issue related to the gene therapy. Last month, Novartis acknowledged the death of a six-month-old patient treated with Zolgensma in the European Phase III clinical trial STRIVE-EU (NCT03461289)—but insisted that the death was not the result of toxicity within the gene therapy. “According to the coroner’s report, the immediate cause of death was hypoxic-ischemic brain damage with respiratory tract infection as the underlying cause,” AveXis stated on September 19. “SMA Type 1 was indicated as the underlying cause for the respiratory tract infection. In addition, there was no evidence of an inflammatory CNS process or a toxic or a treatment-related brain damage.” Zolgensma is an adeno-associated virus vector-based gene therapy that won FDA approval on May 24. Zolgensma is indicated for the treatment of SMA in pediatric patients less than two years of age with SMA with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene.....
|



 Your new post is loading...
Your new post is loading...








Buy Klonopin without prescription
buy liquid morphine online
Buy Morphine online uk
Buy Norco Online Without Prescription
Buy Oramorph without prescription
Buy oxycontin online uk
Buy Quaaludes Online
buy sildenafil 50 mg tablet
Buy yellow Xanax online
Dilaudid for pain
Order Acxion phentermine 30mg
Order codeine without prescription