Your new post is loading...

|
Scooped by
Juan Lama
|
We present early vaccine effectiveness (VE) estimates of the 2023 seasonal COVID-19 vaccination campaign using XBB.1.5 vaccine against COVID-19 hospitalization and ICU admission in previously vaccinated adults ≥60 years old in the Netherlands. We compared vaccination status of 2050 hospitalizations including 92 ICU admissions with age group-, sex-, region- and date-specific population vaccination coverage between 9 October and 5 December 2023. VE against hospitalization was 70.7% (95% CI: 66.6; 74.3), VE against ICU admission was 73.3% (95% CI: 42.2; 87.6). Preprint in medRxiv (Dec. 13, 2023): https://doi.org/10.1101/2023.12.12.23299855

|
Scooped by
Juan Lama
|
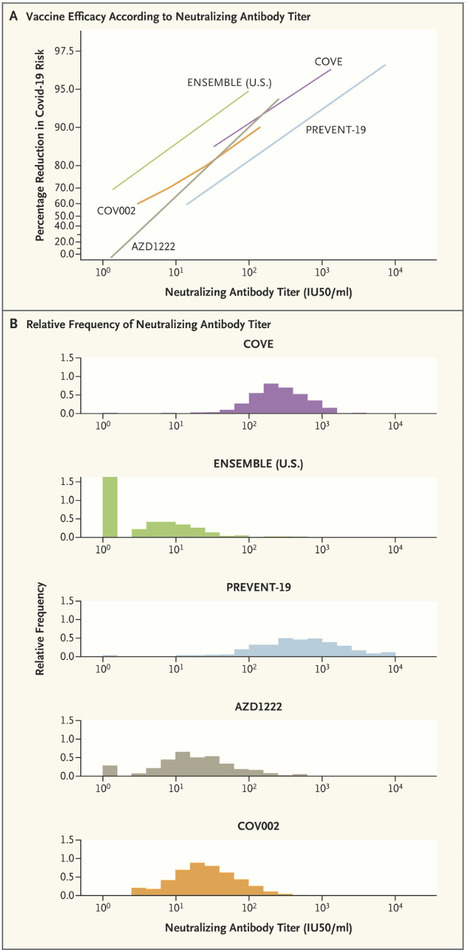
The rapid identification of a correlate of protection (CoP) for Covid-19 vaccines — on the basis of several harmonized randomized phase 3 trials using common validated assays — constitutes an important success in vaccinology. A CoP is an immune marker that can be used to reliably predict a vaccine’s level of efficacy in preventing a clinically relevant outcome. The level of this marker is measured shortly (2 to 4 weeks) after completion of the vaccination regimen and provides an actionable basis for decisions such as regulatory approval of an efficacious vaccine for a new population that was not included in the pivotal randomized phase 3 trials, or approval of a refined version of a vaccine that was previously shown to be efficacious. Once established, a CoP can be used as the primary end point for provisional or full approval of a vaccine for a specific use, if a clinical immunobridging study confirms that high enough levels of the CoP are achieved. For example, the Food and Drug Administration (FDA) extended approval of the mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech) Covid vaccines from older to younger age groups on the basis of a comparison of neutralizing antibody titers. Moreover, FDA guidance and a European Medicines Agency declaration from the International Coalition of Medicines Regulatory Authorities recommended that approval of new vaccine strains and booster doses be based on clinical immunobridging studies showing noninferiority or superiority with respect to a CoP end point. Other applications of a CoP include ensuring vaccine consistency from lot to lot, supporting recommendations for coadministration with other vaccines, and determination of appropriate expiration dates. Confusion about CoPs is understandable, given the myriad complicated issues involved in identifying them and the fact that different uses for CoPs require different validation measures. Evidence that a marker is a CoP is generally derived from five main sources: natural history studies that correlate infection-induced immune responses with outcomes; vaccine-challenge studies in animals or humans; studies that experimentally manipulate the immune marker to directly assess mechanistic causation (e.g., by administering various vaccine doses or using passive antibody transfer); efficacy trials that quantify the relationship between vaccine efficacy and the level of the immune marker in individual vaccine recipients; and meta-analyses of series of efficacy trials that correlate vaccine efficacy with the mean immune-marker level. Strong evidence has been generated from all five of these sources for both serum anti-spike IgG concentration and anti–SARS-CoV-2 neutralizing antibody titer as CoPs for vaccines against symptomatic Covid-19; for brevity, we focus here on the neutralizing antibody titer. Meta-analyses have established high correlations between the standardized mean titer and vaccine efficacy, and the neutralizing antibody titer has consistently been shown to be a mechanistic CoP in challenge studies in nonhuman primates. The U.S. government’s COVID-19 Vaccine Correlates of Protection Program assessed CoPs in phase 3 trials of four vaccines: COVE for mRNA-1273, ENSEMBLE for Ad26.COV2.S, PREVENT-19 for NVX-CoV2373, AZD1222 (United States/Chile/Peru) for ChAdOx1 nCoV-19, and COV002 (United Kingdom) also for ChAdOx1 nCoV-19. Vaccine efficacy always markedly increased with the titer. Published in NEJM (Dec. 10, 2022): https://doi.org/10.1056/NEJMp2211314

|
Scooped by
Juan Lama
|
The vaccine was only about 16 percent effective at reducing a person’s chance of getting a mild or moderate infection, the agency said. Experts said a good rate would be at least 50 percent. This season’s flu vaccine has offered little to no protection against getting a mild or moderate case of influenza, the Centers for Disease Control and Prevention said this week.This season’s flu vaccine has offered little to no protection against getting a mild or moderate case of influenza, the Centers for Disease Control and Prevention said this week. In a study of more than 3,600 Americans in seven states, the C.D.C. said in a report that the vaccine was only around 16 percent effective, a rate that it said was “not statistically significant.” “It’s not ineffective, but it’s clearly suboptimal in its efficacy,” Dr. Jesse L. Goodman, a former chief scientist at the Food and Drug Administration, said on Thursday. He reviewed the report but was not associated with it. Still, despite the vaccine’s lackluster performance this season, which started in October and lasts through May, the C.D.C. suggested that people get inoculated, saying that it could “prevent serious outcomes.” Scientists had warned in 2020 that the flu season, if it was severe, could possibly converge with Covid to create a dreaded “twindemic.” But coronavirus restrictions — including working from home and the use of masks — along with a high flu vaccine rate may have helped reduce caseloads the last few seasons, during which, the C.D.C. said, cases have been at a record low. Still, even a mild flu season can be devastating. The C.D.C. estimated that during the 2019-20 flu season, around 22,000 people in the country had died and 400,000 had been hospitalized. This season, the agency said, “influenza activity” declined in December and January, during the worst of the Omicron surge, but increased in early February. In October and November of 2021, the agency investigated a flu outbreak at the University of Michigan, where there were 745 cases, mostly involving students who had not been vaccinated against the flu. Investigators there also found that the vaccine did not offer much protection. Dr. Goodman said that this season’s results showed how much flu vaccines could be improved. “The next pandemic could be an influenza pandemic,” Dr. Goodman said, “so we need better vaccines.” Every year, scientists decide whether they need to update the flu vaccine to protect against the strains that they predict will dominate the upcoming season. The low efficacy rate this season, Dr. Goodman said, “suggests that there was a mismatch between the strains of virus in the vaccine and what’s circulating.” Scientists updated this season’s vaccines to offer protection against four flu viruses, including H3N2, which ended up being this season’s dominant strain, the report said. H3N2 was also dominant during the 2017-18 flu season, which experts had said was “moderately severe.” Since the agency began calculating the vaccine’s effectiveness in 2004, the efficacy rate has been as high as 60 percent — for the 2010-11 season — and as low as 10 percent, during the first season the C.D.C. tracked it. Dr. Goodman said he would consider a rate between 50 and 80 percent to be good. The flu is a life-threatening respiratory illness that can fill up hospital beds. It shares symptoms with Covid, including fever, coughing, a sore throat and fatigue. Adults 65 and older, those who are pregnant or immunocompromised and children under 5 are most at risk of the flu. CDC report (March 11, 2022) available at: https://www.cdc.gov/mmwr/volumes/71/wr/mm7110a1.htm

|
Scooped by
Juan Lama
|
Background The recently emerged SARS-CoV-2 omicron variant raised concerns around potential escape from vaccine-elicited immunity. Limited data are available on real-world vaccine effectiveness (VE) of mRNA-1273 against omicron. Here, we report VE of 2 or 3 mRNA-1273 doses against infection and hospitalization with omicron and delta, including among immunocompromised individuals. Methods This test negative study was conducted at Kaiser Permanente Southern California. Cases were individuals aged ≥18 years testing positive by RT-PCR with specimens collected between 12/6/2021 and 12/23/2021 with variant determined by spike gene status. Randomly sampled test negative controls were 5:1 matched to cases by age, sex, race/ethnicity, and specimen collection date. Conditional logistic regression models were used to evaluate adjusted odds ratio (aOR) of vaccination with mRNA-1273 doses between cases and controls. VE(%) was calculated as (1-aOR)x100. Results 6657 test positive cases (44% delta, 56% omicron) were included. The 2-dose VE against omicron infection was 30.4% (95% CI, 5.0%-49.0%) at 14-90 days after vaccination and declined quickly thereafter. The 3-dose VE was 95.2% (93.4%-96.4%) against delta infection and 62.5% (56.2%-67.9%) against omicron infection. The 3-dose VE against omicron infection was low among immunocompromised individuals (11.5%; 0.0%-66.5%). None of the cases (delta or omicron) vaccinated with 3 doses were hospitalized compared to 53 delta and 2 omicron unvaccinated cases. Conclusions VE of 3 mRNA-1273 doses against infection with delta was high and durable, but VE against omicron infection was lower. VE against omicron infection was particularly low among immunocompromised individuals. No 3-dose recipients were hospitalized for COVID-19. Preprint available at medRxiv (Jan. 8, 2022): https://doi.org/10.1101/2022.01.07.22268919
|

|
Scooped by
Juan Lama
|
On September 1, 2022, the Moderna and Pfizer–BioNTech bivalent vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) containing equal amounts of spike messenger RNA from the ancestral and omicron BA.4–BA.5 subvariants replaced their monovalent counterparts as booster doses for persons who are 12 years of age or older in the United States. We previously reported surveillance data from North Carolina on the effectiveness of these two bivalent boosters against coronavirus disease 2019 (Covid-19) during the first 3 months after deployment (September 1 to December 8, 2022); the BA.4–BA.5 subvariants were predominant during the first 2.5 months of this period.1 Here, we present two additional months of data that were obtained during a period when the omicron BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants had become predominant to show the durability of protection conferred by these two bivalent boosters against a wider range of clinical outcomes than were included in our previous report. The data sources and study design have been described previously,1-3 and updated information is provided in the Methods section of the Supplementary Appendix, available with the full text of this letter at NEJM.org. The current study used data regarding booster doses and clinical outcomes from September 1, 2022, to February 10, 2023, for all North Carolina residents who were 12 years of age or older. During this period, a total of 6,306,311 residents were eligible to receive bivalent boosters; of these residents, 1,279,802 received the injections. A total of 19,462 of the 154,581 SARS-CoV-2 infections, 253 of the 2208 Covid-19–related hospitalizations, and 79 of the 867 Covid-19–related deaths occurred after receipt of the bivalent booster (Table S1 in the Supplementary Appendix). We considered four outcome measures: infection, severe infection resulting in hospitalization, severe infection resulting in hospitalization or death, and severe infection resulting in death. We fit the Cox regression model with a time-varying hazard ratio for severe infection and fit the proportional-rates model with a time-varying rate ratio for recurrent infection for each additional booster dose that was received (i.e., first booster vs. primary vaccination, second booster vs. first booster, or third booster vs. second booster); all measures were adjusted for the baseline characteristics shown in Table S1. We estimated the booster effectiveness on a particular day as 1 minus the hazard ratio or rate ratio on that day multiplied by 100%. Effectiveness against severe infection resulting in hospitalization was slightly lower, and effectiveness against infection was much lower. The effectiveness against severe infection resulting in death was the highest despite uncertainty because of the small number of events. We also analyzed the data separately for participants who received bivalent boosters before November 1, 2022 (when the BA.4–BA.5 subvariants were predominant) and after November 1, 2022 (when the BQ.1–BQ.1.1 subvariants were more prevalent and then were gradually replaced by the XBB–XBB.1.5 subvariants). The results are shown in the right column of Figure 1 and in Tables S3 and S4. The effectiveness was broadly similar between the two booster cohorts. Finally, we performed subgroup analyses according to the participant’s age and previous infection status and according to the manufacturers of the bivalent vaccine and the previous vaccine. Effectiveness against infection was higher for the Moderna bivalent vaccine than for the Pfizer–BioNTech bivalent vaccine and higher among previously infected participants than among those with no previous infection (Fig. S1). The two types of bivalent boosters were associated with an additional reduction in the incidence of omicron infection among participants who had previously been vaccinated or boosted. Although the two bivalent vaccines were designed to target the BA.4–BA.5 subvariants, they were also associated with a lower risk of infection or severe infection with the BQ.1–BQ.1.1 and XBB–XBB.1.5 subvariants. The effectiveness was higher against hospitalization and death than against infection and waned gradually from its peak over time. Published in NEJM (April 12, 2023): https://doi.org/10.1056/NEJMc2302462

|
Scooped by
Juan Lama
|
The BA.2.75* sublineage of SARS-CoV-2 B.1.1.529 (omicron) variant escapes neutralizing antibodies. We estimated effectiveness of prior infection in preventing reinfection with BA.2.75* using a test-negative, case-control study design. Effectiveness of prior pre-omicron infection against BA.2.75* reinfection, irrespective of symptoms, was 6.0% (95% CI: 1.5-10.4%). Effectiveness of prior BA.1/BA.2 infection was 49.9% (95% CI: 47.6-52.1%) and of prior BA.4/BA.5 infection was 80.6% (95% CI: 71.2-87.0). Effectiveness of prior pre-omicron infection followed by BA.1/BA.2 infection against BA.2.75* reinfection was 56.4% (95% CI: 50.5-61.6). Effectiveness of prior pre-omicron infection followed by BA.4/BA.5 infection was 91.6% (95% CI: 65.1-98.0). Analyses stratified by time since prior infection indicated waning of protection since prior infection. Analyses stratified by vaccination status indicated that protection from prior infection is higher among those vaccinated, particularly among those who combined index-virus-type vaccination with a prior omicron infection. A combination of pre-omicron and omicron immunity is most protective against BA.2.75* reinfection. Viral immune evasion may have accelerated recently to overcome high immunity in the global population, thereby also accelerating waning of natural immunity. Preprint available in medRxiv (Oct. 30, 2022): https://doi.org/10.1101/2022.10.29.22281606

|
Scooped by
Juan Lama
|
BACKGROUND The Ad26.COV2.S vaccine was highly effective against severe–critical coronavirus disease 2019 (Covid-19), hospitalization, and death in the primary phase 3 efficacy analysis. METHODS We conducted the final analysis in the double-blind phase of our multinational, randomized, placebo-controlled trial, in which adults were assigned in a 1:1 ratio to receive single-dose Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe–critical Covid-19 with onset at least 14 days after administration and at least 28 days after administration in the per-protocol population. Safety and key secondary and exploratory end points were also assessed. RESULTS Median follow-up in this analysis was 4 months; 8940 participants had at least 6 months of follow-up. In the per-protocol population (39,185 participants), vaccine efficacy against moderate to severe–critical Covid-19 at least 14 days after administration was 56.3% (95% confidence interval [CI], 51.3 to 60.8; 484 cases in the vaccine group vs. 1067 in the placebo group); at least 28 days after administration, vaccine efficacy was 52.9% (95% CI, 47.1 to 58.1; 433 cases in the vaccine group vs. 883 in the placebo group). Efficacy in the United States, primarily against the reference strain (B.1.D614G) and the B.1.1.7 (alpha) variant, was 69.7% (95% CI, 60.7 to 76.9); efficacy was reduced elsewhere against the P.1 (gamma), C.37 (lambda), and B.1.621 (mu) variants. Efficacy was 74.6% (95% CI, 64.7 to 82.1) against severe–critical Covid-19 (with only 4 severe–critical cases caused by the B.1.617.2 [delta] variant), 75.6% (95% CI, 54.3 to 88.0) against Covid-19 leading to medical intervention (including hospitalization), and 82.8% (95% CI, 40.5 to 96.8) against Covid-19–related death, with protection lasting 6 months or longer. Efficacy against any severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was 41.7% (95% CI, 36.3 to 46.7). Ad26.COV2.S was associated with mainly mild-to-moderate adverse events, and no new safety concerns were identified. CONCLUSIONS A single dose of Ad26.COV2.S provided 52.9% protection against moderate to severe–critical Covid-19. Protection varied according to variant; higher protection was observed against severe Covid-19, medical intervention, and death than against other end points and lasted for 6 months or longer. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722. opens in new tab.) Published in NEJM (Feb. 9, 2022): https://doi.org/10.1056/NEJMoa2117608
|



 Your new post is loading...
Your new post is loading...