Your new post is loading...

|
Scooped by
Juan Lama
|
Cryo-electron microscopy by University of Alabama at Birmingham researchers has exposed the structure of a bacterial virus with unprecedented detail. This is the first structure of a virus able to infect Staphylococcus epidermidis, and high-resolution knowledge of structure is a key link between viral biology and potential therapeutic use of the virus to quell bacterial infections. Bacteriophages or "phages" is the terms used for viruses that infect bacteria. The UAB researchers, led by Terje Dokland, Ph.D., in collaboration with Asma Hatoum-Aslan, Ph.D., at the University of Illinois Urbana-Champaign, have described atomic models for all or part of 11 different structural proteins in phage Andhra. The study is published in Science Advances. Andhra is a member of the picovirus group. Its host range is limited to S. epidermidis. This skin bacterium is mostly benign but also is a leading cause of infections of indwelling medical devices. "Picoviruses are rarely found in phage collections and remain understudied and underused for therapeutic applications," said Hatoum-Aslan, a phage biologist at the University of Illinois. With emergence of antibiotic resistance in S. epidermidis and the related pathogen Staphylococcus aureus, researchers have renewed interest in potentially using bacteriophages to treat bacterial infections. Picoviruses always kill the cells they infect, after binding to the bacterial cell wall, enzymatically breaking through that wall, penetrating the cell membrane and injecting viral DNA into the cell. They also have other traits that make them attractive candidates for therapeutic use, including a small genome and an inability to transfer bacterial genes between bacteria. Knowledge of protein structure in Andhra and understanding of how those structures allow the virus to infect a bacterium will make it possible to produce custom-made phages tailored to a specific purpose, using genetic manipulation. "The structural basis for host specificity between phages that infect S. aureus and S. epidermidis is still poorly understood," said Dokland, a professor of microbiology at UAB and director of the UAB Cryo-Electron Microscopy Core. "With the present study, we have gained a better understanding of the structures and functions of the Andhra gene products and the determinants of host specificity, paving the way for a more rational design of custom phages for therapeutic applications. Our findings elucidate critical features for virion assembly, host recognition and penetration." Staphylococcal phages typically have a narrow range of bacteria they can infect, depending on the variable polymers of wall teichoic acid on the surface of different bacterial strains. "This narrow host range is a double-edged sword: On one hand, it allows the phages to target only the specific pathogen causing the disease; on the other hand, it means that the phage may need to be tailored to the patient in each specific case," Dokland said. The general structure of Andhra is a 20-faced, roundish icosahedral capsid head that contains the viral genome. The capsid is attached to a short tail. The tail is largely responsible for binding to S. epidermidis and enzymatically breaking the cell wall. The viral DNA is injected into the bacterium through the tail. Segments of the tail include the portal from the capsid to the tail, and the stem, appendages, knob and tail tip. The 11 different proteins that make up each virus particle are found in multiple copies that assemble together. For instance, the capsid is made of 235 copies each of two proteins, and the other nine virion proteins have copy numbers from two to 72. In total, the virion is made up of 645 protein pieces that include two copies of a 12th protein, whose structure was predicted using the protein structure prediction program AlphaFold. The atomic models described by Dokland, Hatoum-Aslan, and co-first authors N'Toia C. Hawkins, Ph.D., and James L. Kizziah, Ph.D., UAB Department of Microbiology, show the structures for each protein—as described in molecular language like alpha-helix, beta-helix, beta-strand, beta-barrel or beta-prism. The researchers have described how each protein binds to other copies of that same protein type, such as to make up the hexameric and pentameric faces of the capsid, as well as how each protein interacts with adjacent different protein types. Electron microscopes use a beam of accelerated electrons to illuminate an object, providing much higher resolution than a light microscope. Cryo-electron microscopy adds the element of super-cold temperatures, making it particularly useful for near-atomic structure resolution of larger proteins, membrane proteins or lipid-containing samples like membrane-bound receptors, and complexes of several biomolecules together. In the past eight years, new electron detectors have created a tremendous jump in resolution for cryo-electron microscopy over normal electron microscopy. Key elements of this so-called "resolution revolution" for cryo-electron microscopy are: - Flash-freezing aqueous samples in liquid ethane cooled to below -256 degrees F. Instead of ice crystals that disrupt samples and scatter the electron beam, the water freezes to a window-like "vitreous ice."
- The sample is kept at super-cold temperatures in the microscope, and a low dose of electrons is used to avoid damage to the proteins.
- Extremely fast direct electron detectors are able to count individual atoms at hundreds of frames per second, allowing sample movement to be corrected on the fly.
- Advanced computing merges thousands of images to generate three-dimensional structures at high resolution. Graphics processing units are used to churn through terabytes of data.
- The microscope stage that holds the sample can also be tilted as images are taken, allowing construction of a three-dimensional tomographic image, similar to a CT scan at the hospital.
The analysis of Andhra virion structure by the UAB researchers started with 230,714 particle images. Molecular reconstruction of the capsid, tail, distal tail and tail tip started with 186,542, 159,489, 159,489 and 159,489 images, respectively. Resolution ranged from 3.50 to 4.90 angstroms. Cited research published (Nov. 30, 2022) in Science Advances: https://doi.org/10.1126/sciadv.ade0459

|
Scooped by
Juan Lama
|
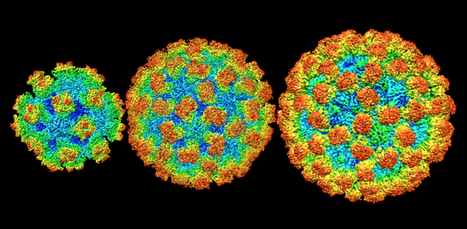
A new structural analysis of four norovirus strains reveals that the virus’s shell varies in size and molecular arrangement – a surprise finding that could help scientists developing vaccines. Norovirus, best known for sweeping through daycares and cruise ships, can form small, medium, and large structures depending on the viral strain. The discovery, reported June 10, 2019, in the journal Proceedings of the National Academy of Sciences, overturns nearly two decades of conventional wisdom about norovirus. Until now, the only structural data about the virus that scientists had came from a single, not particularly prevalent, strain. “Everyone thought that all the strains would look about the same – like the one that was solved 20 years ago,” says Howard Hughes Medical Institute Investigator Leemor Joshua-Tor. “It turns out that they don’t!” Joshua-Tor’s team used a microscopy technique called cryo-electron microscopy (cryo-EM) to visualize the shells of four viral strains, including one responsible for up to roughly 80 percent of norovirus outbreaks. That strain was 71 percent larger (by volume) than the one previously reported. Its shell was also decorated with a different pattern of molecular spikes. Those structural details will be crucial for scientists working on vaccines or antiviral therapies to treat norovirus infection, says Joshua-Tor, a structural biologist at Cold Spring Harbor Laboratory (CSHL). Though norovirus causes about 21 million cases of foodborne illness in the United States every year, there are currently no approved therapies. At least one vaccine candidate is working its way through clinical trials now. But CSHL study coauthor James Jung says scientists will need to take the virus’s newfound variation into account – so any new vaccine protects against a broad array of strains. HHMI talked with Jung and Joshua-Tor to find out more about the “stomach flu,” the virus that causes it, and how they finally solved the structure of outbreak strains’ shells...... Published in PNAS on June 25, 2019: https://doi.org/10.1073/pnas.1903562116
|

|
Scooped by
Juan Lama
|
Structural-biology method crosses a key resolution threshold. A founding principle of structural biology is that, once researchers can directly observe macromolecules in enough detail, it should be possible to understand how their 3D structures confer their biological functions. Indeed, many scientific advances have relied on directly observing the world around us in as much detail as possible, and efforts are increasingly being dedicated to visualizing the atomic structures of biological components that have a key role in human disease. Nuclear magnetic resonance (NMR) spectroscopy, X-ray crystallography and cryo-electron microscopy (cryo-EM) are the three main structural-biology techniques in use. Of the three, cryo-EM has emerged as the current ‘go to’ method for determining the structures of large and dynamic complexes that have proved difficult to obtain by the other approaches. Writing in Nature, Yip et al.1 and Nakane et al.2 report the sharpest images yet obtained by using a method termed single-particle cryo-EM, enabling the location of individual atoms in a protein to be determined for the first time. Breakthroughs reported by other groups have also produced notable improvements in the resolution of cryo-EM images3,4. Ultimately, these developments will help researchers gain a better understanding, at unprecedented resolution, of how proteins work in health and disease, with the potential to aid the design of better therapeutics. Although cryo-EM is a decades-old technique, it has garnered increasing interest since around 2013 due to a series of technological and algorithmic advances that together drove a striking improvement in the resolution obtainable by this technique (described as the ‘resolution revolution’) Collecting single-particle cryo-EM data begins with a protein sample that has been applied to a special sample grid. Plunging it into liquid ethane flash-freezes and traps the protein particles in a thin film of amorphous ice. Two-dimensional images of the individual particles in the sample grid, obtained by applying a beam of electrons, are averaged computationally to yield a 3D structure. The 2D images are incredibly ‘noisy’ because a low dose of electrons must be used to avoid damaging the radiation-sensitive biological sample. As such, these images have historically been unsuitable for determining structures at an atomic level of detail. However, the advances reported since 2013 have allowed single-particle cryo-EM data to be collected that rival those obtained using X-ray crystallography. The resolution revolution of cryo-EM has continued to advance6. Yip et al. and Nakane et al. harnessed technological improvements to determine the structures of a stable iron-storing protein called ferritin (termed apoferritin in the absence of metals) to a resolution of approximately 1.2 ångströms. These structures are the highest-resolution single-particle cryo-EM reconstructions so far determined, and the data are of sufficiently high quality to resolve the individual atoms in apoferritin (Fig. 1). This unprecedented feat would not have been thought feasible merely a decade ago....

|
Scooped by
Juan Lama
|
A new type of vaccine that can be stored at warmer temperatures, removing the need for refrigeration, has been developed for mosquito-borne virus Chikungunya in a major advance in vaccine technology. The findings, published in Science Advances today [Wednesday 25 September], reveal exceptionally promising results for the Chikungunya vaccine candidate, which has been engineered using a synthetic protein scaffold that could revolutionise the way vaccines are designed, produced and stored. Researchers from the University of Bristol and the French National Centre for Scientific Research (CNRS) in Grenoble, France, teamed up with computer technology giant Oracle to find a way to make vaccines that are thermostable (able to withstand warm temperatures), can be designed quickly and are easily produced. “We were working with a protein that forms a multimeric particle resembling a virus but is completely safe, because it has no genetic material inside, said Pascal Fender, expert virologist at CNRS. “Completely by chance, we discovered that this particle was incredibly stable even after months, without refrigeration.” “This particle has a very flexible, exposed surface that can be easily engineered, added Imre Berger, Director of the Max Planck-Bristol Centre for Minimal Biology in Bristol. “We figured that we could insert small, harmless bits of Chikungunya to generate a virus-like mimic we could potentially use as a vaccine.” To validate their design, the scientists employed cryo-electron microscopy, a powerful new technique recently installed in Bristol’s state-of-the-art microscopy facility headed by Christiane Schaffitzel, co-author of the study. Cryo-EM yields very large data sets from which the structure of a sample can be determined at near atomic resolution, requiring massive parallel computing. Enabled by Oracle’s high-performance cloud infrastructure, the team developed a novel computational approach to create an accurate digital model of the synthetic vaccine. University of Bristol IT specialists Christopher Woods and Matt Williams, together with colleagues at Oracle, implemented software packages seamlessly on the cloud in this pioneering effort. Christopher explained: “We were able to process the large data sets obtained by the microscope on the cloud in a fraction of the time and at much lower cost than previously thought possible.” “Researchers have had a long tradition of building and installing their own super computers on-premises, but cloud computing is allowing them to run large data sets in record time, with fast connectivity and low latency. This is helping them crunch data and make scientific breakthroughs much faster. Going forward, technologies like machine learning and cloud computing will play a significant part in the scientific world, and we are delighted we could help the researchers with this important discovery,” added Phil Bates, leading cloud architect at Oracle. The particles the scientists designed yielded exceptionally promising results in animal studies, soundly setting the stage for a future vaccine to combat Chikungunya disease.... Published on September 25, 2019 in Science Advances (Open Access): https://doi.org/10.1126/sciadv.aaw2853
|



 Your new post is loading...
Your new post is loading...