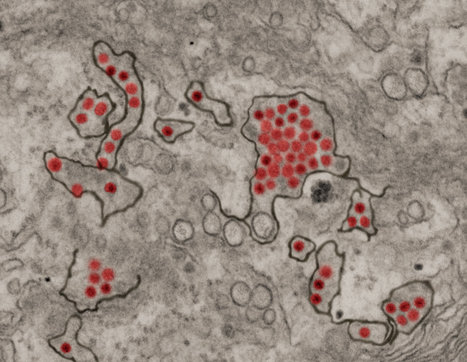
A protein that is critical in controlling replication of West Nile and Zika viruses—and could be important for developing therapies to prevent and treat those viruses—has been identified by a Georgia State University biologist and his research group.
The researchers found Z-DNA binding protein 1 (ZBP1) is a sensor that plays a significant role in triggering a robust immune response when it detects a viral infection within cells. The Georgia State study, published in the journal Frontiers in Microbiology, found ZBP1 is essential for restricting both West Nile and Zika virus replication, and that it prevents West Nile-associated encephalitis (inflammation of the brain) in mice. The absence of ZBP1 in mice leads to 100 percent mortality when infected with even a non-disease-producing strain of West Nile Virus, the study found. "It's significant because you take a virus that has never been shown to kill anything and if you block this protein the virus will just kill everything," said Mukesh Kumar, assistant professor of biology and senior author of the study. "We discovered that when cells are infected with viruses such as Zika and West Nile, they respond by triggering necroptosis, a form of programmed cell death, via ZBP1 signaling. This inhibits viral replication and spread, allowing the immune system to clear the virus."
Kumar said the findings could present new treatment strategies for viruses that can infect the central nervous system by modulating ZBP1 expression. Subsequent research by Kumar's team will explore effectiveness against similar viruses such as Eastern Equine Encephalitis and Powassan virus. West Nile Virus is the leading mosquito-born disease and cause of viral encephalitis in the United States, with more than 50,000 people affected, including 480 cases reported in Georgia, according to the Centers for Disease Control and Prevention. There have been 2,330 associated deaths since it first reached the country in 1999. The Culex species of mosquito responsible for spreading it is common throughout the world.
Zika, which is spread by the Aedes mosquito that has been found as far north as Florida and Texas, can cause serious neurological diseases such as Guillain-Barre syndrome, which causes the body's immune system to attack the nervous system. Birth defects such as microcephaly, an abnormally small head and brain can result. Most people who get Zika or West Nile don't get sick thanks to the body's natural immune response and may not know they've been infected, meaning their cases probably don't get reported. Of the West Nile cases reported in the U.S., nearly 50 percent invade the nervous system leading to life-threatening or -altering consequences such as encephalitis. Drug treatments are often ineffective once a virus reaches the brain, but Kumar hopes enhancing host ZBP1 expression within the central nervous system could clear the virus from the brain and prevent severe disease associated with neuroinvasive viral infections such as West Nile and Zika....
Study Published in Frontiers in Microbiology (Sept. 11, 2019):
https://doi.org/10.3389/fmicb.2019.02089



 Your new post is loading...
Your new post is loading...