Your new post is loading...

|
Scooped by
Juan Lama
|
The Orthocoronavirinae subfamily is large comprising four highly divergent genera. Four seasonal coronaviruses were circulating in humans prior to the coronavirus disease 2019 pandemic. Infection with these viruses induced antibody responses that are relatively narrow with little cross-reactivity to spike proteins of other coronaviruses. Here, we report that infection with and vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces broadly cross-reactive binding antibodies to spikes from a wide range of coronaviruses including members of the sarbecovirus subgenus, other betacoronaviruses including Middle Eastern respiratory syndrome coronavirus, and extending to alpha-, gamma-, and delta-coronavirus spikes. These data show that the coronavirus spike antibody landscape in humans has profoundly been changed and broadened as a result of the SARS-CoV-2 pandemic. While we do not understand the functionality of these cross-reactive antibodies and their impact, there is the possibility that they may lead to enhanced resistance of the population to infection with newly emerging coronaviruses with pandemic potential. IMPORTANCE As demonstrated by severe acute respiratory syndrome coronavirus 2, coronaviruses pose a significant pandemic threat. Here, we show that coronavirus disease 2019 mRNA vaccination can induce significant levels of cross-reactive antibodies against diverse coronavirus spike proteins. While these antibodies are binding antibodies that likely have little neutralization capacity and while their contribution to cross-protection is unclear, it is possible that they may play a role in protection from progression to severe disease with novel coronaviruses. Published in mBio (Dec. 19, 2023):

|
Scooped by
Juan Lama
|
In a recent study posted to the bioRxiv* preprint server, researchers analyzed the viral genomes of 28 endemic viruses to study the evolution of the ability of viruses to evade the neutralizing antibodies elicited by vaccines or previous infections. Background Viruses evolve rapidly and adapt to changing environments due to their high mutation rates and low generation time. Often viruses adapted to different animal hosts infect humans and optimize the methods through which they enter and replicate in the host cell, increasing the human-to-human transmission and evolving into a novel pathogen. The early stages of pandemics are often characterized by high adaptive evolutionary rates, as was seen during the coronavirus disease 2019 (COVID-19) pandemic and outbreaks related to various other respiratory viruses. While some viruses become endemic after adapting to a new host and do not evolve further, other endemic viruses continue to adapt through antigenic evolution, resulting in an arms race between the virus and the human immune system. Since viruses that undergo antigenic evolution pose the risk of repeat infections and increase their ability to evade vaccine-induced immunity, understanding which viruses continue to undergo antigen evolution could help manage future disease outbreaks. About the study The present study used sequence data for each gene in 28 viral genomes to estimate adaptive evolutionary rates. These 28 viruses spanned ten families and were transmitted between humans through various modes. Viruses with potentially high antigenic evolution rates were identified based on the high evolutionary rates for the genes coding for receptor-binding proteins since the receptor-binding region is involved in antibody neutralization and harbors most mutations that allow antigenic escape. The adaptive evolutionary rates were calculated in terms of the number of adaptive mutations in each codon per year, which allowed the adaptive evolutionary rates to be compared across the various genes in the genome and across viruses. The adaptive evolutionary rates of three viruses that were known to undergo antigenic evolution — coronavirus 229E, influenza viruses A/H3N2, and influenza viruses B/Yam — were compared against the evolutionary rates of three antigenically stable viruses, hepatitis A, measles, and influenza C/Yamagata. To understand the patterns of adaptive evolution in recent years, the sequence data for 28 viruses that are pathogenic to humans were obtained and curated. These viruses included deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) viruses and were transmitted between humans through bodily fluids, blood, vectors, fecal-oral, and respiratory routes. The researchers only investigated endemic viruses since they were interested in understanding the antigenic evolution that occurs during the endemic phase and not the initial adaptive phase. The evolutionary rates of the receptor binding protein, which was expected to be highly variable across endemic viruses, and the polymerase gene, which was expected to be conserved, were compared across the 28 viruses. Since the evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been relatively recent, and the Omicron variant carried a large number of mutations indicating a single fixation event, SARS-CoV-2 was compared with ten other antigenically evolving viruses by comparing the amino-acid substitution rates in the receptor binding protein. Results The results reported that 10 of the 28 viruses undergo adaptive evolution resulting in the antigenic mutations that allow the viruses to escape the immunity induced by previous infections and vaccines. Furthermore, comparing amino-acid substitution rates between SARS-CoV-2 and other viruses revealed that SARS-CoV-2 is evolving and accumulating mutations that cause protein-coding changes at rates much higher than other endemic viruses. Antigenic evolution was found to be more prevalent in RNA viruses. Still, the researchers believe that since the list of viruses included in the study was not comprehensive and comprised only well-studied viruses, determining the proportion of antigenically evolving endemic viruses is difficult. Furthermore, the rate of adaptive mutations might not reflect the phenotypic changes occurring in the viruses, implying that viruses with receptor-binding protein genes that evolve at the same rates might not develop the ability to evade vaccine or infection-induced immunity at the same rates. Conclusions Overall, the findings suggested that many human viruses that have become endemic continue to evolve antigenically, gaining the ability to escape the neutralizing antibodies generated by vaccinations or previous infections. Ten of the 28 viruses investigated in this study showed continued adaptive evolution. In contrast, the amino-acid substitution rates showed that SARS-CoV-2 is evolving faster than the other ten endemic human viruses. Cited ressearch available in bioRxiv (May 22, 2023): https://doi.org/10.1101/2023.05.19.541367

|
Scooped by
Juan Lama
|
In comparing breakthrough infections from the SARS-CoV-2 Delta and Omicron variants, the latter, though milder than Delta infections, were associated with lower antibody titers and limited cross-neutralizing immunity, suggesting reduced protection against re-infection or infection from a future variant. Summary Virus-like particle (VLP) and live virus assays were used to investigate neutralizing immunity against Delta and Omicron SARS-CoV-2 variants in 259 samples from 128 vaccinated individuals. Following Delta breakthrough infection, titers against WT rose 57-fold and 3.1-fold compared to uninfected boosted and unboosted individuals, respectively, versus only a 5.8-fold increase and 3.1-fold decrease for Omicron breakthrough infection. Among immunocompetent, unboosted patients, Delta breakthrough infections induced 10.8-fold higher titers against WT compared to Omicron (p=0.037). Decreased antibody responses in Omicron breakthrough infections relative to Delta were potentially related to a higher proportion of asymptomatic or mild breakthrough infections (55.0% versus 28.6%, respectively), which exhibited 12.3-fold lower titers against WT compared to moderate-severe infections (p=0.020). Following either Delta or Omicron breakthrough infection, limited variant-specific cross-neutralizing immunity was observed. These results suggest that Omicron breakthrough infections are less immunogenic than Delta, thus providing reduced protection against reinfection or infection from future variants. Highlights 1. In breakthrough infections, variant-specific cross-neutralizing immunity is limited. 2. Higher antibody titers are observed in severe versus mild breakthrough infections. 3. Delta breakthroughs exhibited 10.8X higher antibody titers compared to Omicron. 4. The rise in antibody titers from Omicron breakthroughs was 1/3 of that from boosting. Published in Cell (March 17, 2022): https://doi.org/10.1016/j.cell.2022.03.019

|
Scooped by
Juan Lama
|
It’s not yet clear whether the antibody levels produced will lead to immunity to the virus. To prove that, the companies will need to conduct large studies. An experimental Covid-19 vaccine being developed by the drug giant Pfizer and the biotech firm BioNTech spurred immune responses in healthy patients, but also caused fever and other side effects, especially at higher doses. The first clinical data on the vaccine were disclosed Wednesday in a paper released on medRXiv, a preprint server, meaning it has not yet been peer-reviewed or published in a journal. “We still have a ways to go and we’re testing other candidates as well,” said Philip Dormitzer, the chief scientific officer for viral vaccines at Pfizer’s research laboratories. “However, what we can say at this point is there is a viable candidate based on immunogenicity and early tolerability safety data.” The study randomly assigned 45 patients to get one of three doses of the vaccine or placebo. Twelve received a 10-microgram dose, 12 a 30-microgram dose, 12 a 100-microgram dose, and nine a placebo. The 100-microgram dose caused fevers in half of patients; a second dose was not given at that level. Following a second injection three weeks later of the other doses, 8.3% of the participants in the 10-microgram group and 75% of those in the 30-microgram group developed fevers. More than 50% of the patients who received one of those doses reported some kind of adverse event, including fever and sleep disturbances. None of these side effects was deemed serious, meaning they did not result in hospitalization or disability and were not life-threatening. The vaccine generated antibodies against SARS-CoV-2, the virus that causes Covid-19, and some of these antibodies were neutralizing, meaning that they appear to prevent the virus from functioning. Levels of neutralizing antibodies were 1.8 to 2.8 times the level of that in the recovered patients. It’s not certain that higher antibody levels will lead to immunity to the virus. To prove that, Pfizer will need to conduct large studies that aim to prove that people who have received the vaccine are at least 50% less likely to become infected. Those studies are expected to begin this summer, mostly in the United States. Pfizer and BioNTech are testing four different versions of the vaccine, but only one will advance to larger studies.... Preprint available at medRxiv (July 1, 2020): https://doi.org/10.1101/2020.06.30.20142570

|
Scooped by
Juan Lama
|
Scientists in Shanghai say some recovered patients show no signs of the neutralising proteins. Researchers in Shanghai hope to determine whether some recovered coronavirus patients have a higher risk of reinfection after finding surprisingly low levels of Covid-19 antibodies in a number of people discharged from hospital. A team from Fudan University analysed blood samples from 175 patients discharged from the Shanghai Public Health Clinical Centre and found that nearly a third had unexpectedly low levels of antibodies. In some cases, antibodies could not be detected at all. “Whether these patients were at high risk of rebound or reinfection should be explored in further studies,” the team wrote in preliminary research released on Monday on Medrxiv.org, an online platform for preprint papers. Although the study was preliminary and not peer-reviewed, it was the world’s first systematic examination of antibody levels in patients who had recovered from Covid-19, the disease caused by the coronavirus, the researchers said. All of the patients had recently recovered from mild symptoms of the disease and most of those with low antibody levels were young. The researchers excluded patients who had been admitted to intensive care units because many of them already had antibodies from donated blood plasma.... Preprint of the original Report in medRxiv (April 6, 2020):
|

|
Scooped by
Juan Lama
|
COVID-19 vaccines have recently been updated with the spike protein of SARS-Co-V-2 XBB.1.5 subvariant alone, but their immunogenicity in humans has yet to be fully evaluated and reported, particularly against emergent viruses that are rapidly expanding. We now report that administration of an updated monovalent mRNA vaccine (XBB.1.5 MV) to uninfected individuals boosted serum virus-neutralization antibodies significantly against not only XBB.1.5 (27.0-fold) and the currently dominant EG.5.1 (27.6-fold) but also key emergent viruses like HV.1, HK.3, JD.1.1, and JN.1 (13.3-to-27.4-fold). In individuals previously infected by an Omicron subvariant, serum neutralizing titers were boosted to highest levels (1,764-to-22,978) against all viral variants tested. While immunological imprinting was still evident with the updated vaccines, it was not nearly as severe as the previously authorized bivalent BA.5 vaccine. Our findings strongly support the official recommendation to widely apply the updated COVID-19 vaccines to further protect the public. Preprint in bioRxiv (Nov. 27, 2023): https://doi.org/10.1101/2023.11.26.568730

|
Scooped by
Juan Lama
|
In this study, by characterizing several human monoclonal antibodies (mAbs) isolated from single B cells of the COVID-19–recovered individuals in India who experienced ancestral Wuhan strain (WA.1) of SARS-CoV-2 during early stages of the pandemic, we found a receptor binding domain (RBD)–specific mAb 002-S21F2 that has rare gene usage and potently neutralized live viral isolates of SARS-CoV-2 variants including Alpha, Beta, Gamma, Delta, and Omicron sublineages (BA.1, BA.2, BA.2.12.1, BA.4, and BA.5) with IC50 ranging from 0.02 to 0.13 μg/ml. Structural studies of 002-S21F2 in complex with spike trimers of Omicron and WA.1 showed that it targets a conformationally conserved epitope on the outer face of RBD (class 3 surface) outside the ACE2-binding motif, thereby providing a mechanistic insights for its broad neutralization activity. The discovery of 002-S21F2 and the broadly neutralizing epitope it targets have timely implications for developing a broad range of therapeutic and vaccine interventions against SARS-CoV-2 variants including Omicron sublineages. Published in Science Advances (October 5, 2022): https://doi.org/10.1126/sciadv.add2032

|
Scooped by
Juan Lama
|
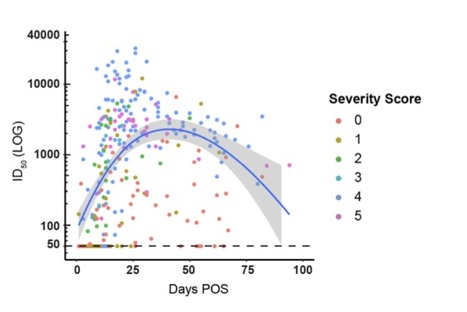
Antibody (Ab) responses to SARS-CoV-2 can be detected in most infected individuals 10-15 days following the onset of COVID-19 symptoms. However, due to the recent emergence of this virus in the human population it is not yet known how long these Ab responses will be maintained or whether they will provide protection from re-infection. Using sequential serum samples collected up to 94 days post onset of symptoms (POS) from 65 RT-qPCR confirmed SARS-CoV-2-infected individuals, we show seroconversion in >95% of cases and neutralizing antibody (nAb) responses when sampled beyond 8 days POS. We demonstrate that the magnitude of the nAb response is dependent upon the disease severity, but this does not affect the kinetics of the nAb response. Declining nAb titres were observed during the follow up period. Whilst some individuals with high peak ID50 (>10,000) maintained titres >1,000 at >60 days POS, some with lower peak ID50 had titres approaching baseline within the follow up period. A similar decline in nAb titres was also observed in a cohort of seropositive healthcare workers from Guy′s and St Thomas′ Hospitals. We suggest that this transient nAb response is a feature shared by both a SARS-CoV-2 infection that causes low disease severity and the circulating seasonal coronaviruses that are associated with common colds. This study has important implications when considering widespread serological testing, Ab protection against re-infection with SARS-CoV-2 and the durability of vaccine protection. Preprint available at medRxiv (July 11, 2020): https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1

|
Scooped by
Juan Lama
|
The vaccine from Inovio Pharmaceuticals is designed to work by injecting synthetic DNA that codes for protective antibodies. novio Pharmaceuticals on Tuesday said that its investigational Covid-19 vaccine had “positive” results in a small trial. But the company, which has gained more than $4 billion in value since the coronavirus pandemic began, provided none of the details necessary to determine whether the vaccine is working. In a press release, Inovio said its vaccine led to “immunological response rates” in 34 of 36 patients in the trial, but did not disclose how many patients produced antibodies that neutralize the coronavirus — data key to determining whether the vaccine could protect against infection. The company did not immediately respond to a request for more information. The company’s press release appeared to play down the importance of neutralizing antibodies, pointing to a study that found roughly one-third of patients who recovered from Covid-19 had no detectable antibodies in their blood. Kathryn Edwards, scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn., said Inovio’s vaccine appears to be safe enough to merit further study, but without more data on patient responses, it’s impossible to say whether it might have any beneficial effect. Inovio was early to announce plans to develop a vaccine against the novel coronavirus, boosting its stock price tenfold before producing any clinical data. Its enterprise value started the year at $300 million but grew to $4.5 billion on the hope that its coronavirus vaccine work would lead to a successful product. The stock fell 13% to $27.50 in early Tuesday trading following the release of the early vaccine results. Press Release available (June 30, 2020): http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Positive-Interim-Phase-1-Data-For-INO-4800-Vaccine-for-COVID-19/default.aspx

|
Scooped by
Juan Lama
|
A collaborative team of scientists has made a successful proof-of-principle demonstration of an advanced HIV vaccine strategy--an approach that may also work in protecting people from an array of other deadly infectious diseases. The team, led by scientists at Scripps Research, also included the Ragon Institute of Massachusetts General Hospital, MIT and Harvard University; the La Jolla Institute for Immunology; and IAVI, a scientific research organization focused on HIV and other global health challenges. Their research appears in Science. The new vaccine strategy centers on stimulating the immune system to produce broadly neutralizing antibodies (bnAbs) against HIV. These special antibodies are capable of neutralizing many different strains of the fast-mutating virus by binding to important yet difficult-to-access regions of the virus surface that don't vary much from strain to strain. The new vaccine strategy centers on stimulating the immune system to produce broadly neutralizing antibodies (bnAbs) against HIV. These special antibodies are capable of neutralizing many different strains of the fast-mutating virus by binding to important yet difficult-to-access regions of the virus surface that don't vary much from strain to strain. A vaccine that elicits such antibodies could save many millions of lives and billions of dollars--and ultimately, may help eliminate HIV as a significant public health problem. Based on a concept called "germline targeting," this novel strategy could potentially provide protection against the millions of different strains of the virus circulating globally. Achieving this goal has so far been elusive; no HIV vaccine candidate has ever been shown to induce a protective bnAb response in humans. "I believe that we need a germline-targeting strategy to develop an effective vaccine against HIV, and the same type of strategy may be helpful for making vaccines against many other difficult pathogens," says the study's co-senior author William Schief, PhD, a professor in the Department of Immunology and Microbiology at Scripps Research. "Here, with a great collaborative effort among multiple labs, we've shown the feasibility of a general germline-targeting approach." This study drew scientists from diverse backgrounds and areas of expertise: co-senior authors are Scripps Research's Schief, Facundo Batista, PhD, chief scientific officer at the Ragon Institute, and Shane Crotty, PhD, a professor in the Vaccine Discovery Division at La Jolla Institute for Immunology. The germline-targeting approach is meant to launch the production of a desired bnAb by stimulating the right antibody-producing cells. Antibodies are produced by immune cells called B cells, which start out in a "naïve" or "germline" state. A large repertoire of these germline B cells circulates in the blood and other tissues. In a viral infection--or after immunization with a vaccine that mimics an infecting virus--some germline B cells will bind at least weakly to structures on the surface of the virus. That will stimulate the cells to begin a weeks-long maturation process, in which the antibodies continuously improve in their ability to bind to the surface, thereby neutralizing the virus. The germline-targeting strategy for an HIV vaccine aims to stimulate the small number of germline B cells that are capable of maturing into cells that make bnAbs. Researchers suspect that other attempts to create an HIV vaccine that elicits bnAbs have failed because they haven't stimulated a sufficient number of these "bnAb precursor" germline B cells. Schief and colleagues previously demonstrated a germline-targeting strategy for one special case: a bnAb that grabs hold of HIV in an unusual way. The new approach is more powerful because it works for antibodies that grip their targets via a much more common mechanism. Furthermore, analyses performed in the study indicate that the approach can likely also be applied to vaccines for many other difficult pathogens such as influenza, dengue virus, Zika virus, hepatitis C and malaria. To demonstrate the feasibility of their strategy, Schief and Jon Steichen, PhD, a study co-first author and senior scientist in the Schief lab, started by choosing a known HIV bnAb called BG18 as the test case. Informed by structural studies of BG18 bound to its target on the virus--including structures determined for this study in the lab of Andrew Ward, PhD, professor of Integrative Structural and Computational Biology at Scripps Research, and published structures from the lab of Pamela Bjorkman, PhD, at Caltech--Steichen and Schief identified key features of this antibody's HIV-gripping ability. Next, they searched a large database of human antibody genes in order to find B cells making antibodies that naturally share BG18's key features. Then they used a sophisticated strategy to select and evolve a set of virus-mimicking proteins that could potentially activate multiple BG18-like B cells. These proteins would eventually serve as "immunogens" to stimulate BG18-like B cells in human vaccination. "As the repertoire of B cells differs from person to person, and in the same person over time, we believe that you need to target more than a few of these cells to have a reasonable chance of activating one of them in any given vaccine recipient," Steichen says. In the lab of Shane Crotty at the La Jolla Institute for Immunology, tests of blood samples from HIV-negative human donors confirmed that the team's immunogens bound well to normal circulating B cells that have the desired BG18-like features..... Published in Science (October 31, 2019): https://doi.org/10.1126/science.aax4380
|




 Your new post is loading...
Your new post is loading...