Your new post is loading...

|
Scooped by
Juan Lama
|
By applying machine learning to medical record data, scientists found that secondary bacterial pneumonia that does not resolve was a key driver of death in patients with COVID-19. It may even exceed death rates from the viral infection itself. The scientists also found evidence that COVID-19 does not cause a “cytokine storm,” so often believed to cause death. "Our study highlights the importance of preventing, looking for and aggressively treating secondary bacterial pneumonia in critically ill patients with severe pneumonia, including those with COVID-19," said senior author Dr. Benjamin Singer, an associate professor of medicine at Northwestern University Feinberg School of Medicine and a Northwestern Medicine pulmonary and critical care physician. The investigators found nearly half of patients with COVID-19 develop a secondary ventilator-associated bacterial pneumonia. “Those who were cured of their secondary pneumonia were likely to live, while those whose pneumonia did not resolve were more likely to die,” Singer said. “Our data suggested that the mortality related to the virus itself is relatively low, but other things that happen during the ICU stay, like secondary bacterial pneumonia, offset that.” The study findings also negate the cytokine storm theory, said Singer, also the Lawrence Hicks Professor of Pulmonary Medicine at Feinberg. “The term ‘cytokine storm’ means an overwhelming inflammation that drives organ failure in your lungs, your kidneys, your brain and other organs,” Singer said. “If that were true, if cytokine storm were underlying the long length of stay we see in patients with COVID-19, we would expect to see frequent transitions to states that are characterized by multi-organ failure. That’s not what we saw.” The study analyzed 585 patients in the intensive care unit (ICU) at Northwestern Memorial Hospital with severe pneumonia and respiratory failure, 190 of whom had COVID-19. The scientists developed a new machine learning approach called CarpeDiem, which groups similar ICU patient-days into clinical states based on electronic health record data. This novel approach, which is based on the concept of daily rounds by the ICU team, allowed them to ask how complications like bacterial pneumonia impacted the course of illness. These patients or their surrogates consented to enroll in the Successful Clinical Response to Pneumonia Therapy (SCRIPT) study, an observational trial to identify new biomarkers and therapies for patients with severe pneumonia. As part of SCRIPT, an expert panel of ICU physicians used state-of-the-art analysis of lung samples collected as part of clinical care to diagnose and adjudicate the outcomes of secondary pneumonia events. “The application of machine learning and artificial intelligence to clinical data can be used to develop better ways to treat diseases like COVID-19 and to assist ICU physicians managing these patients,” said study co-first author Dr. Catherine Gao, an instructor in pulmonary and critical care medicine at Feinberg and a Northwestern Medicine physician. “The importance of bacterial superinfection of the lung as a contributor to death in patients with COVID-19 has been underappreciated because most centers have not looked for it or only look at outcomes in terms of presence or absence of bacterial superinfection, not whether treatment is successful or not,” said study co-author Dr. Richard Wunderink, who leads the Successful Clinical Response in Pneumonia Therapy Systems Biology Center at Northwestern. The next step in the research will be to use molecular data from the study samples and integrate it with machine learning approaches to understand why some patients go on to be cured of pneumonia and some don’t. Investigators also want to expand the technique to larger datasets and use the model to make predictions that can be brought back to the bedside to improve the care of critically ill patients. Other Northwestern authors on the paper include Nikolay S. Markov, Thomas Stoeger, Anna E. Pawlowski,Mengjia Kang,Prasanth Nannapaneni, Rogan A. Grant,Chiagozie Pickens, James M. Walter, Jacqueline M. Kruser, Luke V. Rasmussen, Daniel Schneider, Justin Starren, Helen K. Donnelly, Alvaro Donayre, Yuan Luo, Scott Budinger and Alexander Misharin. The study was supported by the Simpson Querrey Lung Institute for Translational Sciences and grant U19AI135964 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Study Published in the J. Clin. Investig. (April 27, 2023): https://doi.org/10.1172/JCI170682

|
Scooped by
Juan Lama
|
Authorities in Kazakhstan have denied a report published by Chinese officials that the country is experiencing an outbreak of "unknown pneumonia" potentially deadlier than the novel coronavirus. On Thursday, the Chinese Embassy in Kazakhstan issued a warning to citizens living in the Central Asian country that the pneumonia had killed more than 1,700 people. "Kazakhstani Health Department and other agencies are conducting comparative research and have not defined the nature of the pneumonia virus," the statement said. New cases of the unidentified pneumonia have been increasing significantly since mid-June across the country, said the embassy, adding that in some places, authorities are reporting hundreds of new cases a day. In a statement on Friday, the Kazakhstan health ministry acknowledged the presence of "viral pneumonias of unspecified etiology," but denied that the outbreak was new or unknown. "In response to these reports, the Ministry of Health of the Republic of Kazakhstan officially declares that this information does not correspond to reality," the statement read. It added the "unspecified" pneumonia classification followed World Health Organization guidelines "for the registration of pneumonia when the coronavirus infection is diagnosed clinically or epidemiologically but is not confirmed by laboratory testing." According to the embassy, the rise was concentrated in the regions of Atyrau, Aktobe and Shymkent, which together have almost 500 new cases and more than 30 critically ill patients. The disease killed 1,772 people this year, some of whom were Chinese citizens, according to the embassy. A total of 628 of those deaths took place in June alone. "This disease is much deadlier than Covid-19," the statement said....

|
Scooped by
Juan Lama
|
Aims: Studies have indicated that chloroquine (CQ) shows antagonism against COVID-19 in vitro. However, evidence regarding its effects in patients is limited. This study aims to evaluate the efficacy of hydroxychloroquine (HCQ) in the treatment of patients with COVID-19. Main methods: From February 4 to February 28, 2020, 62 patients suffering from COVID-19 were diagnosed and admitted to Renmin Hospital of Wuhan University. All participants were randomized in a parallel-group trial, 31 patients were assigned to receive an additional 5-day HCQ (400 mg/d) treatment, Time to clinical recovery (TTCR), clinical characteristics, and radiological results were assessed at baseline and 5 days after treatment to evaluate the effect of HCQ. Key findings: For the 62 COVID-19 patients, 46.8% (29 of 62) were male and 53.2% (33 of 62) were female, the mean age was 44.7 (15.3) years. No difference in the age and sex distribution between the control group and the HCQ group. But for TTCR, the body temperature recovery time and the cough remission time were significantly shortened in the HCQ treatment group. Besides, a larger proportion of patients with improved pneumonia in the HCQ treatment group (80.6%, 25 of 31) compared with the control group (54.8%, 17 of 31). Notably, all 4 patients progressed to severe illness that occurred in the control group. However, there were 2 patients with mild adverse reactions in the HCQ treatment group. Significance: Among patients with COVID-19, the use of HCQ could significantly shorten TTCR and promote the absorption of pneumonia. Preprint available at medRxiv since March 31, 2020: https://doi.org/10.1101/2020.03.22.20040758

|
Scooped by
Juan Lama
|
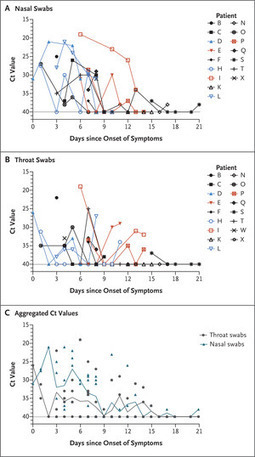
From January 7 through January 26, 2020, a total of 14 patients who had recently returned from Wuhan and had fever (≥37.3°C) received a diagnosis of Covid-19 (the illness caused by SARS-CoV-2) by means of reverse-transcriptase–polymerase-chain-reaction assay with primers and probes targeting the N and Orf1b genes of SARS-CoV-2; the assay was developed by the Chinese Center for Disease Control and Prevention. Samples were tested at the Guangdong Provincial Center for Disease Control and Prevention. Thirteen of 14 patients with imported cases had evidence of pneumonia on computed tomography (CT). None of them had visited the Huanan Seafood Wholesale Market in Wuhan within 14 days before symptom onset. Patients E, I, and P required admission to intensive care units, whereas the others had mild-to-moderate illness. Secondary infections were detected in close contacts of Patients E, I, and P. Patient E worked in Wuhan and visited his wife (Patient L), mother (Patient D), and a friend (Patient Z) in Zhuhai on January 17. Symptoms developed in Patients L and D on January 20 and January 22, respectively, with viral RNA detected in their nasal and throat swabs soon after symptom onset. Patient Z reported no clinical symptoms, but his nasal swabs (cycle threshold [Ct] values, 22 to 28) and throat swabs (Ct values, 30 to 32) tested positive on days 7, 10, and 11 after contact. A CT scan of Patient Z that was obtained on February 6 was unremarkable. Patients I and P lived in Wuhan and visited their daughter (Patient H) in Zhuhai on January 11 when their symptoms first developed. Fever developed in Patient H on January 17, with viral RNA detected in nasal and throat swabs on day 1 after symptom onset.... We analyzed the viral load in nasal and throat swabs obtained from the 17 symptomatic patients in relation to day of onset of any symptoms (Figure 1C). Higher viral loads (inversely related to Ct value) were detected soon after symptom onset, with higher viral loads detected in the nose than in the throat. Our analysis suggests that the viral nucleic acid shedding pattern of patients infected with SARS-CoV-2 resembles that of patients with influenza4 and appears different from that seen in patients infected with SARS-CoV.3 The viral load that was detected in the asymptomatic patient was similar to that in the symptomatic patients, which suggests the transmission potential of asymptomatic or minimally symptomatic patients. These findings are in concordance with reports that transmission may occur early in the course of infection5 and suggest that case detection and isolation may require strategies different from those required for the control of SARS-CoV. How SARS-CoV-2 viral load correlates with culturable virus needs to be determined. Identification of patients with few or no symptoms and with modest levels of detectable viral RNA in the oropharynx for at least 5 days suggests that we need better data to determine transmission dynamics and inform our screening practices.
|

|
Scooped by
Juan Lama
|
BACKGROUND The efficacy and safety of tofacitinib, a Janus kinase inhibitor, in patients who are hospitalized with coronavirus disease 2019 (Covid-19) pneumonia are unclear. METHODS We randomly assigned, in a 1:1 ratio, hospitalized adults with Covid-19 pneumonia to receive either tofacitinib at a dose of 10 mg or placebo twice daily for up to 14 days or until hospital discharge. The primary outcome was the occurrence of death or respiratory failure through day 28 as assessed with the use of an eight-level ordinal scale (with scores ranging from 1 to 8 and higher scores indicating a worse condition). All-cause mortality and safety were also assessed. RESULTS A total of 289 patients underwent randomization at 15 sites in Brazil. Overall, 89.3% of the patients received glucocorticoids during hospitalization. The cumulative incidence of death or respiratory failure through day 28 was 18.1% in the tofacitinib group and 29.0% in the placebo group (risk ratio, 0.63; 95% confidence interval [CI], 0.41 to 0.97; P=0.04). Death from any cause through day 28 occurred in 2.8% of the patients in the tofacitinib group and in 5.5% of those in the placebo group (hazard ratio, 0.49; 95% CI, 0.15 to 1.63). The proportional odds of having a worse score on the eight-level ordinal scale with tofacitinib, as compared with placebo, was 0.60 (95% CI, 0.36 to 1.00) at day 14 and 0.54 (95% CI, 0.27 to 1.06) at day 28. Serious adverse events occurred in 20 patients (14.1%) in the tofacitinib group and in 17 (12.0%) in the placebo group. CONCLUSIONS Among patients hospitalized with Covid-19 pneumonia, tofacitinib led to a lower risk of death or respiratory failure through day 28 than placebo. (Funded by Pfizer; STOP-COVID ClinicalTrials.gov number, NCT04469114. opens in new tab.) Published in NEJM (June 16, 2021): https://doi.org/10.1056/NEJMoa2101643

|
Scooped by
Juan Lama
|
Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) is mainly spread through respiratory droplets or direct contact. But the infection condition of genital system is still unknown. This study aimed to evaluate whether or not SARS-CoV-2 is found in the vaginal fluid of women with COVID-19 illness. 10 women with confirmed severe COVID-19 pneumonia admitted to in Tongji Zhongfa Hospital Intensive care union(ICU) ward from Feb 4, 2020 to Feb 24, 2020 were included. Clinical records, laboratory results, and computer tomography(CT)-scan examination were retrospectively reviewed. The evidence of genital infection potential was accessed by testing for the presence of SARS-CoV-2 in vaginal fluids obtained from vaginal swab samples. Reverse transcriptase polymerase chain reaction(RT-PCR) was used to confirm the SARS-CoV-2 infection in vaginal fluids. The clinical characteristics of these ten women were similar to those reported severe COVID-19 patients. All ten patients were tested for SARS-CoV-2 in vaginal fluid, and all samples tested negative for the virus. Findings from this small group of cases suggest that no SARS-CoV-2 virus existing in the vaginal fluids of severe COVID-19 patients. Published in Clinical Infectious Diseases (April 2, 2020): https://doi.org/10.1093/cid/ciaa375

|
Scooped by
Juan Lama
|
A new AI-powered diagnosis system promises to detect new coronavirus cases with an accuracy rate of up to 96% via computerized tomographyscans, Chinese tech outlet Sina Tech News reported. The diagnosis algorithm was developed by Alibaba's research institute Damo Academy. Researchers at the academy said they had trained the AI model with sample data from more than 5,000 confirmed cases, adding that the system could identify differences in CT scans between patients infected with the novel virus and those with ordinary viral pneumonia with an accuracy of up to 96%. The algorithm included the latest treatment guidelines and recently published research, said its creators. The new diagnostic tool was first introduced in the new Qiboshan Hospital in Zhengzhou, Henan province, which was modeled on Beijing's Xiaotangshan Hospital, completed in 2003 to deal with the SARS crisis. The new hospital started accepting patients infected with coronavirus on Sunday. The system would also be adopted in more than 100 hospitals in the provinces of Hubei, Guangdong and Anhui, said Alibaba. The new algorithm could alleviate pressure on hospitals, as it can complete the recognition process within 20 seconds, according to Alibaba. Usually, it will take a doctor between five and 15 minutes to analyze a CT scan of one suspected patient and give a clinical diagnosis, with scans sometimes including more than 300 images. The Chinese National Health Commission on Feb. 5 widened the criteria for the diagnosis of new infections, adding CT scan results on top of the previous nucleic acid test method, to ensure patients with clinical symptoms will receive standard treatment as soon as possible. This new diagnosis system is not Alibaba's first attempt to use AI to combat coronavirus. Researchers from Damo Academy have developed an AI-powered public health service tool that provides information related to the SARS-CoV-2 coronavirus, which was first deployed by the government of Zhejiang province on Jan. 27. The system can answer most inquiries regarding the pandemic via an app.
|



 Your new post is loading...
Your new post is loading...