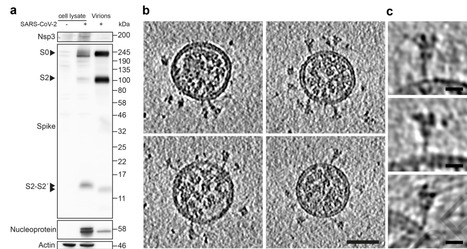
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virions are surrounded by a lipid bilayer from which spike (S) protein trimers protrude. Heavily glycosylated S trimers bind the ACE2 receptor and mediate entry of virions into target cells. S exhibits extensive conformational flexibility: it modulates exposure of its receptor binding site and later undergoes complete structural rearrangement to drive fusion of viral and cellular membranes. The structures and conformations of soluble, overexpressed, purified S proteins have been studied in detail using cryo-electron microscopy.
The structure and distribution of S on the virion surface, however, has not been characterized. Here we applied cryo-electron microscopy and tomography to image intact SARS-CoV-2 virions, determining the high-resolution structure, conformational flexibility and distribution of S trimers in situ on the virion surface. These results reveal the conformations of S present on the virion, and provide a basis from which to understand interactions between S and neutralizing antibodies during infection or vaccination.
Published in Nature (August 17, 2020):



 Your new post is loading...
Your new post is loading...