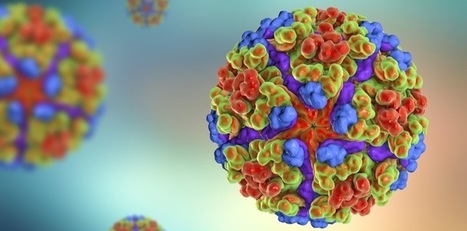
The French vaccine developer Valneva has received a €21M ($23.4M) grant to catch up with the competition in commercializing the first potential vaccine for the chikungunya virus. Coming from the nonprofit Coalition for Epidemic Preparedness Innovations (CEPI), the grant will boost the manufacturing and development of Valneva’s vaccine for the tropical disease chikungunya, which causes fever and can trigger chronic pain in the joints. Chikungunya has long been a problem in developing countries with endemic tropical diseases, but has received less attention from developed countries until recently. However, climate change could increase the range of disease-carrying mosquitoes, and chikungunga outbreaks in Europe could become more common. Companies developing vaccines for the disease could deliver an important weapon in the fight against the disease.
In May, the vaccine showed the potential to protect against the virus in a phase I trial. As the company has already calculated an optimal dose, Valneva’s one-shot vaccine could skip phase II and head directly to phase III.
The Austrian biotech Themis Bioscience is also developing a vaccine for chikungunya, which is expected to enter phase III later this year. With a phase III trial expected to begin in early 2020, Valneva might narrow Themis’ lead in the race to get the first chikungunya vaccine to the market.
The CEPI allocates grants to fund the development of vaccines against infections causing humanitarian crises. CEPI also recently provided a 19M euros grant to Themis, with the aim of accelerating vaccines for chikungunya and other diseases to the market.



 Your new post is loading...
Your new post is loading...