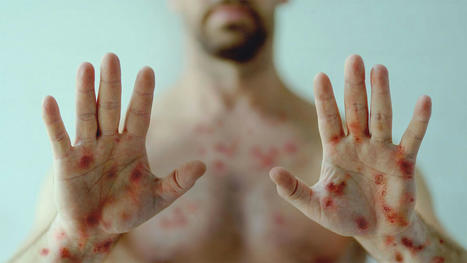
Reports of myocarditis, penile necrosis, and atypical/persistent rash requiring amputation. Be alert for potentially severe manifestations of monkeypox in patients who are immunocompromised or co-infected with HIV, the CDC told healthcare workers on Thursday. "People who are immunocompromised due to HIV or other conditions are at higher risk for severe manifestations of monkeypox than people who are immunocompetent," the agency said in a health advisory to its Health Alert Network, adding that "the HIV status of all sexually active adults and adolescents with suspected or confirmed monkeypox should be determined." Of the patients with severe manifestations "for whom CDC has been consulted," the majority had HIV with CD4 counts below 200 cells/mL, indicating "substantial immunosuppression," the agency noted. These severe manifestations have included cases involving atypical or persistent rash requiring amputation of an extremity; secondary bacterial or fungal infections; lesions in sensitive areas resulting in severe pain or urethral or bowel strictures; and comorbidities involving organ systems, including myocarditis, transverse myelitis, bowel lesions, penile necrosis, and others. The news arrives as the U.S. records its third known monkeypox-associated death in Ohio. To date, over 25,000 monkeypox cases have been reported in the U.S., 38% of which involved patients co-infected with HIV. Researchers have highlighted monkeypox severity and outcomes in people with HIV before.
For example, a study in The Lancet about a 2017-2018 monkeypox outbreak in Nigeria reported seven deaths in a population of 122 patients, with four of the seven co-infected with HIV. More recently, papers on the current outbreak have reported conflicting results about the association between monkeypox severity and HIV co-infection. CDC data involving more than 1,300 monkeypox patients showed a higher rate of hospitalizations for those with HIV (8% vs 3% for those without HIV), with higher rates for those whose HIV was not virally suppressed. Conversely, German researchers reported that the clinical characteristics in HIV-infected versus non-infected monkeypox patients were largely similar. The new CDC advisory recommends that monkeypox treatment include optimization of immune function for people with immunocompromising conditions, such as limiting the use of immunosuppressive medications when "not otherwise clinically indicated," and offering antiretroviral therapy for those living with HIV.
Severe immunocompromising conditions can including those with autoimmune disease where immunodeficiency is a clinical component; leukemia or lymphoma, transplant recipients; and those treated with high-dose steroids, alkylating agents, antimetabolites, radiation, or tumor necrosis factor (TNF) inhibitors. On a case-by-case basis, medications such as oral and intravenous tecovirimat (Tpoxx), cidofovir or brincidofovir, and vaccinia immune globulin intravenous should be considered, "although there are no data on effectiveness in treating human monkeypox with these medical countermeasures," according to the CDC. Clinicians should gather repeat lesion swabs in patients with persistent monkeypox DNA, and continue tecovirimat beyond 14 days (but not more than 90 days), "until there is clinical improvement," the advisory stated. Modifications to the dose, frequency, and duration of tecovirimat may be necessary, depending on the patient's clinical condition, disease progression, or therapeutic response. The advisory encouraged clinicians to consult with the CDC Monkeypox Response Clinical Escalations team when appropriate. The following severe manifestations seen in monkeypox patients were reported to the CDC, although the advisory did not include information about HIV status:
- Atypical or persistent rash with coalescing or necrotic lesions, or both, some of which have required extensive surgical debridement or amputation of an affected extremity
- Lesions on a significant proportion of the total body surface area, which may be associated with edema and secondary bacterial or fungal infections among other complications
- Lesions in sensitive areas (including mucosal surfaces such as the oropharynx, urethra, rectum, and vagina) resulting in severe pain that interferes with activities of daily living
- Bowel lesions that are exudative or cause significant tissue edema, leading to obstruction
- Severe lymphadenopathy that can be necrotizing or obstructing (such as in airways)
- Lesions leading to stricture and scar formation resulting in significant morbidity such as urethral and bowel strictures, phimosis, and facial scarring
The CDC advisory also noted reports of individuals with severe monkeypox involving multiple organ systems and associated comorbidities, including oropharyngeal lesions inhibiting oral intake; pulmonary involvement with nodular lesions; neurologic conditions, including encephalitis and transverse myelitis; cardiac complications, including myocarditis and pericardial disease; ocular conditions including severe conjunctivitis; sight-threatening corneal ulcerations; and urologic involvement including urethritis and penile necrosis. The advisory also urged healthcare providers and public health jurisdictions to encourage patients to enroll in the AIDS Clinical Trials Group STOMP trial to evaluate the efficacy of tecovirimat for monkeypox.
CDC Health Advisory published September 29, 2022:
https://emergency.cdc.gov/han/2022/han00475.asp



 Your new post is loading...
Your new post is loading...









https://emergency.cdc.gov/han/2022/han00475.asp