Your new post is loading...

|
Scooped by
Juan Lama
|
WASHINGTON — The Food and Drug Administration on Wednesday authorized the first redesign of coronavirus vaccines since they were rolled out in late 2020, setting up millions of Americans to receive new booster doses targeting Omicron subvariants as soon as next week. The agency cleared two options aimed at the BA.5 variant of Omicron that is now dominant: one made by Pfizer and its German partner BioNTech for use in people as young as 12, and the other by Moderna, for those 18 and older. The doses can be given at least two months since people last received a booster dose or completed their initial series of vaccinations. Biden administration officials have argued that even as researchers work to understand how protective the new shots might be, inoculating Americans again in the coming weeks could help curb the persistently high number of infections and deaths. “As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose,” Dr. Robert M. Califf, the F.D.A. commissioner, said in a statement on Wednesday. He added that the vaccine would “provide better protection against currently circulating variants.” An average of about 90,000 infections and 475 deaths are recorded every day around the United States, almost three years into a pandemic that has killed more than a million Americans and driven a historic drop in life expectancy. But there are also hopeful signs. Even with high case counts, fewer than 40,000 people are currently hospitalized with the virus, a decrease of 10 percent since early August and far fewer than during the Delta-driven surge last summer or the Omicron-fueled wave last winter. Deaths have also remained somewhat flat in recent weeks, a sign that vaccines are helping to prevent the worst outcomes of Covid-19. Ample evidence suggests that many Americans will hold back from getting the updated boosters, either because they are weary of the pandemic or may not feel urgency about an additional dose. With each new shot offered, there are fewer takers. As more companies bring workers back to offices and students return to campuses this fall, persuading Americans to get the updated booster shots will be a major challenge for the administration. The companies produced the retooled shots with extraordinary speed, a testament to the mRNA technology that Pfizer and Moderna have harnessed since the early months of the coronavirus outbreak. The Food and Drug Administration advised companies only two months ago on the formulation that they should adopt for the new vaccines. By later this week, millions of those doses will be delivered to states. The tight timeline meant that the companies went to federal regulators this summer with more limited data on the redesigned boosters than a traditional review process would call for, generating some controversy. Regulators authorized the vaccine without results from human trials, which have just started. Federal officials argue that because the coronavirus is evolving so quickly, human trials would be out of date before they can deliver results that could be used in the F.D.A.’s authorization decision. Instead, they are relying on the results of mouse trials and earlier human trials by Pfizer and Moderna of reformulations aimed at previous versions of the virus. The Biden administration is casting the shots as a standard upgrade that Americans should embrace ahead of potential surges in cases in the winter, like the flu shot, which is reconfigured every year to target more current versions of the influenza virus. The boosters are arriving during a period when the White House has been largely quiet on the pandemic. President Biden has rarely commented on the coronavirus in recent months, even after he tested positive in July. The White House no longer holds regular news briefings on the federal pandemic response, as it did in the first year of the administration — a reflection of the weariness of many Americans in keeping up with Covid precautions. “Covid-19 is the third leading cause of death in the United States. And it’s as if we’ve just accepted that that is going to be the case,” said Mercedes Carnethon, an epidemiologist at Northwestern University’s Feinberg School of Medicine. “I really hope as many people as possible will seek the updated booster so we can protect those who will have a terrible outcome.” Vaccinations remain the cornerstone of the federal government’s Covid strategy, even with tests and treatments widely available. The Biden administration has ordered over 170 million doses for the fall campaign, and officials do not expect shortages when they are rolled out. “If it’s freezing cold out and you have children, you’re going to dress them warmly. This is the concept here,” said Dr. Paul G. Auwaerter, the clinical director of the infectious diseases division at the Johns Hopkins University School of Medicine. “You’ll want to head into the respiratory season with a virus that we know has surprised us with a booster.” Exactly how protective the shots might be is unknown, Dr. Auwaerter said. He pointed to the modest increases in neutralizing antibodies that the companies found in vaccines they tested this year that targeted the original form of Omicron. How antibody levels would translate to protection with the new vaccines was unclear, he added. Experts warned against trying to choose Moderna’s shot over Pfizer’s or vice versa; with research in humans just beginning, scientists are months from knowing whether one brand offers better protection than the other. Many Americans have recently been infected with variants in the Omicron family and have some protection from their bouts with the virus, a development that federal agencies may take into account when recommending how the new shots are used. An advisory committee to the Centers for Disease Control and Prevention is scheduled to meet this week to make recommendations. “For most people, the risk of death is so low at this point, because they’ve gotten infected or vaccinated, or more likely both,” said Dr. Gregory A. Poland, a professor of medicine and infectious diseases and the director of the Vaccine Research Group at the Mayo Clinic. Dr. Poland, who has advised Moderna, Pfizer and White House officials on coronavirus vaccines, said updating booster shots the way the Food and Drug Administration did on Wednesday amounted to a “chase your tail” strategy, tweaking the design incrementally to try to keep up with a fast-changing virus. The new boosters, he said, could potentially save some lives among the elderly and those with immune deficiencies. But they were unlikely to make as substantial an impact with the rest of the population.

|
Scooped by
Juan Lama
|
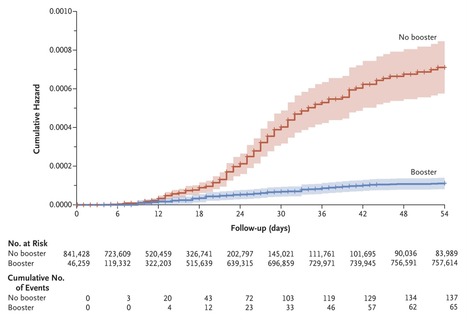
BACKGROUND The emergence of the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 and the reduced effectiveness over time of the BNT162b2 vaccine (Pfizer–BioNTech) led to a resurgence of coronavirus disease 2019 (Covid-19) cases in populations that had been vaccinated early. On July 30, 2021, the Israeli Ministry of Health approved the use of a third dose of BNT162b2 (booster) to cope with this resurgence. Evidence regarding the effectiveness of the booster in lowering mortality due to Covid-19 is still needed. METHODS We obtained data for all members of Clalit Health Services who were 50 years of age or older at the start of the study and had received two doses of BNT162b2 at least 5 months earlier. The mortality due to Covid-19 among participants who received the booster during the study period (booster group) was compared with that among participants who did not receive the booster (nonbooster group). A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of booster status with death due to Covid-19, with adjustment for sociodemographic factors and coexisting conditions. RESULTS A total of 843,208 participants met the eligibility criteria, of whom 758,118 (90%) received the booster during the 54-day study period. Death due to Covid-19 occurred in 65 participants in the booster group (0.16 per 100,000 persons per day) and in 137 participants in the nonbooster group (2.98 per 100,000 persons per day). The adjusted hazard ratio for death due to Covid-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% confidence interval, 0.07 to 0.14; P<0.001). CONCLUSIONS Participants who received a booster at least 5 months after a second dose of BNT162b2 had 90% lower mortality due to Covid-19 than participants who did not receive a booster. Published in NEJM (Dec. 8, 2021): https://doi.org/10.1056/NEJMoa2115624

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration cleared a path for millions more Americans to receive Covid-19 vaccine booster shots, as the nation looks to bolster its defenses and prevent another virus surge. The agency said in a statement on Wednesday that Moderna Inc. vaccine recipients 65 and over can receive a third shot, as can adults 18 and up who are at high risk of severe Covid or with frequent institutional or occupational exposure to the virus that causes the disease. Additionally, all J&J recipients 18 and older are eligible for a booster shot at least two months after receiving their first dose. The agency also allowed each of the available Covid vaccines to be used as a booster dose for eligible individuals following completion of a primary vaccination with a different vaccine. The moves will mean the U.S. has a bigger toolkit to try to limit a potential winter virus rebound. The summer’s delta-variant fueled spike in infections helped increase urgency to make boosters available, and health officials across the U.S. are eager to forestall a rebound in cases that could cripple hospitals and disrupt work and school this winter. FDA officials indicated they would also move quickly to expand eligibility for booster shots as more data become available or if breakthrough cases start to rise in younger adults. “We will not hesitate to drop this age range as we see that that benefit clearly outweighs the risk,” said Peter Marks, the head of the agency’s Center for Biologics Evaluation and Research, during a media briefing following the announcement. The clearances came after a panel of expert advisers to the FDA unanimously backed the Moderna and J&J booster regimens in two days of meetings last week. Regulators have now signed off on boosters for all three coronavirus vaccines available in the U.S. Last month, the FDA said people 65 and over and others who are at heightened risk of severe Covid were eligible for a booster dose of the vaccine developed by Pfizer Inc. and BioNTech SE. Moderna shares climbed 0.8% in after-hours trading in New York, while J&J shares gained 0.4% and Pfizer shares rose 0.2%. U.S.-traded shares of Germany-based BioNTech gained 0.9%. Smaller Dose The Moderna booster shot authorized by the FDA is half the dose that is given in the initial two-shot series, and it should be given at least six months after the initial inoculation, regulators said. The FDA said that a single booster dose of the Pfizer vaccine may be given at least 6 months after completing the primary series to people 18 to 64 with frequent institutional or occupational exposure to the coronavirus. In permitting mixing and matching, the FDA is allowing J&J vaccine recipients to receive an additional dose of any cleared vaccine after two months. Likewise, recipients of Moderna and Pfizer who are eligible for a booster would receive their booster, including J&J’s shot, at least six months after their initial immunization regimen. Marks said during the call with reporters that different combinations produce different antibody levels in the short term, but it isn’t clear what that means in terms of actual long-term protection. Seen as a convenient, effective alternative to two-shot messenger RNA vaccines, J&J’s single-shot immunization has seen far less use in the U.S., in part because it isn’t as effective. The drugmaker has also experienced manufacturing problems that limited the shot’s distribution. The decision to allow mixing will create greater flexibility and is beneficial to global public health, Paul Stoffels, J&J’s chief scientific officer, said in a statement. Before the Moderna and J&J booster shots can be administered, the Centers for Disease Control and Prevention’s Advisory Panel on Immunization Practices will make further recommendations about who should receive them. The panel is scheduled to discuss boosters on Thursday. The next big milestone for the U.S. immunization effort looms next week, when the FDA advisory panel is expected to weigh Pfizer’s proposed Covid vaccine for children ages 5 to 11. If authorized, it could begin to roll out to pediatricians’ offices and drugstores as soon as next month. — With assistance by Jeannie Baumann, and Riley Griffin FDA Press Release (Oct. 20, 2021) available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-additional-actions-use-booster-dose-covid-19-vaccines

|
Scooped by
Juan Lama
|
People who’ve received a third dose of a Covid-19 vaccine booster are reporting rates of side effects similar to those after the second dose, according to data released Tuesday by the Centers for Disease Control and Prevention. The new report, published in the Morbidity and Mortality Weekly Report, relies on submissions from thousands of people who received third shots of the mRNA vaccines from Pfizer-BioNTech and Moderna after such doses were authorized for people with compromised immune systems. People submitted their reactions to v-safe, the CDC’s smartphone-based surveillance network. Among more than 12,500 people who completed surveys after each shot, 79.4% of people reported local reactions (including itching, pain, or redness at the injection site), while 74.1% reported systemic reactions (mostly fatigue, muscle aches, and headaches), typically the day after the shot. That compared to 77.6% and 76.5% of the people who reported local or systemic reactions, respectively, after their second shot. Overall, among more than 22,000 v-safe registrants who’ve received third doses, “no unexpected patterns of adverse reactions were observed,” the report says. As of Sept. 19, some 2.2 million people in the United States had received additional doses of the Covid-19 vaccines. The report focuses on the more common, less worrisome side effects of the mRNA vaccines, and doesn’t mention the very rare, but more serious events like anaphylaxis. Some vaccine side effects occur so infrequently that even studies of thousands of people don’t detect them; it’s only after the shots are administered into millions of arms that a connection becomes clear. But the new data provide at least an early hint that in terms of immediate reactions, it doesn’t seem as if third shots cause more of a kick than the second shots did. Gathering such data is important as the government authorizes boosters for wider populations. Last week, the CDC recommended boosters for people 65 and older, adults with certain underlying health issues, and those who work places or living sites place them at higher risk of coronavirus exposure. So far, those recommendations only apply for people who received the Pfizer vaccine as their initial series, but boosters for the Moderna and Johnson & Johnson shots are expected to eventually be authorized. “These initial findings indicate no unexpected patterns of adverse reactions after an additional dose of Covid-19 vaccines; most of these adverse reactions were mild or moderate,” the authors wrote in the new report. The authors note that enrollment in v-safe is voluntary and is “likely not representative of the vaccinated U.S. population,” with the majority of participants identifying as white. Cited findings published in MMWR (Sept. 28, 2021): http://dx.doi.org/10.15585/mmwr.mm7039e4

|
Scooped by
Juan Lama
|
WASHINGTON (AP) — Dealing the White House a stinging setback, a government advisory panel overwhelmingly rejected a plan Friday to give Pfizer COVID-19 booster shots across the board, and instead endorsed the extra dose only for those who are 65 or older or run a high risk of severe disease. The twin votes represented a heavy blow to the Biden administration’s sweeping effort, announced a month ago, to shore up nearly all Americans’ protection amid the spread of the highly contagious delta variant. The decision was made by an influential committee of outside experts who advise the Food and Drug Administration. In a surprising turn, the panel rejected, by a vote of 16-2, boosters for almost everyone. Members cited a lack of safety data on extra doses and also raised doubts about the value of mass boosters, rather than ones targeted to specific groups. Then, in an 18-0 vote, it endorsed the extra shot for select portions of the U.S. population — namely, those most at risk from the virus. That would help salvage part of the White House’s campaign but would still be a huge step back from the far-reaching plan proposed by the administration a month ago to offer booster shots of both the Pfizer and Moderna vaccines to practically everybody eight months after they get their second dose. Friday’s vote was just the first step in the process. The FDA itself is expected to make a decision on boosters in the next few days, but it usually follows the committee’s recommendations. The offering of boosters is also subject to approval by the Centers for Disease Control and Prevention. A CDC advisory panel is expected to take up the question on Wednesday. The CDC has said it is considering boosters for older people, nursing home residents and front-line health care workers, rather than all adults. Separate FDA and CDC decisions will be needed in order for people who received the Moderna or J&J shots to get boosters. During several hours of vigorous debate Friday, members of the panel questioned the value of offering boosters to almost everybody 16 and over. “I don’t think a booster dose is going to significantly contribute to controlling the pandemic,” said Dr. Cody Meissner of Tufts University. “And I think it’s important that the main message we transmit is that we’ve got to get everyone two doses.” Dr. Amanda Cohn of the CDC said: “At this moment it is clear that the unvaccinated are driving transmission in the United States.” Scientists inside and outside the government have been divided in recent days over the need for boosters and who should get them, and the World Health Organization has strongly objected to rich nations giving a third round of shots when poor countries don’t have enough vaccine for their first. While research suggests immunity levels in those who have been vaccinated wane over time and boosters can reverse that, the Pfizer vaccine is still highly protective against severe illness and death, even amid the spread of the highly contagious delta variant. The surprise turn of events could reinforce recent criticism that the Biden administration got out ahead of the science in its push for boosters. President Joe Biden promised early on that his administration would “follow the science,” following disclosures of political meddling in the Trump administration’s coronavirus response. The FDA panel’s overwhelming initial rejection came despite full-throated arguments about the need for boosters from both Pfizer and health officials from Israel, which began offering boosters to its citizens in July. Sharon Alroy-Preis of Israel’s Ministry of Health said the booster dose improves protection tenfold against infection in people 60 and older. “It’s like a fresh vaccine,” bringing protection back to original levels and helping Israel “dampen severe cases in the fourth wave,” she said. And representatives for Pfizer argued that it is important to shore up immunity before protection against severe disease starts to erode. A company study of 44,000 people showed effectiveness against symptomatic COVID-19 was 96% two months after the second dose, but had dropped to 84% by around six months. Both Pfizer and the Israeli representatives faced pushback from panelists. Several were skeptical about the relevance of Israel’s experience to the U.S. Another concern was whether third doses would exacerbate serious side effects. Meissner said he was worried about extra doses for younger age groups, given the risk of heart inflammation that has been seen in mostly younger men after a second dose. While the condition is very rare, he said, it is not clear if that risk would increase with another dose. Pfizer pointed to Israeli data from nearly 3 million boosters to suggest side effect rates would be similar to that seen after second doses. Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia, said he was supportive of a third dose for adults over 60 or 65, but “I really have trouble” supporting it for anyone down to age 16. While an extra shot likely will at least temporarily decrease cases with mild or no symptoms, “the question becomes what will be the impact of that on the arc of the pandemic, which may not be all that much,” Offit said. Biden’s top health advisers, including the heads of the FDA and CDC, first announced plans for widespread booster shots in mid-August, targeting the week of Sept. 20 as an all-but-certain start date. But that was before FDA staff scientists had completed their own assessments of the data. Earlier this week, two top FDA vaccine reviewers joined a group of international scientists in publishing an editorial rejecting the need for boosters in healthy people. The scientists said continuing studies show the shots are working well despite the delta variant. On Friday, U.S. Surgeon General Dr. Vivek Murthy said that in announcing its booster plan, the Biden administration was not trying to pressure regulators to act but was instead trying to be transparent with the public and be prepared in the event that extra shots won approval. “We have always said that this initial plan would be contingent on the FDA and the CDC’s independent evaluation,” Murthy said. The Biden plan has also raised major ethical concerns about impoverished parts of the world still clamoring for vaccine. But the administration has argued that the plan is not an us-or-them choice, noting that the U.S. is supplying large quantities of vaccine to the rest of the globe. The U.S. has already approved Pfizer and Moderna boosters for certain people with weakened immune systems, such as cancer patients and transplant recipients. Some Americans, healthy or not, have managed to get boosters, in some cases simply by showing up and asking for a shot. And some health systems already are offering extra doses to high-risk people.

|
Scooped by
Juan Lama
|
The authorization applies to people who received solid organ transplants and others with similarly compromised immune systems. The Food and Drug Administration on Thursday authorized third doses of Pfizer-BioNTech’s and Moderna’s coronavirus vaccines for some people with weakened immune systems, giving physicians more leeway to protect those who did not respond enough to an initial series of shots. The authorization, in the form of updates to the existing emergency use authorizations for the two vaccines, applies to people who received solid organ transplants and others with similarly compromised immune systems, the F.D.A. said. The agency’s decision came a day before the Centers for Disease Control and Prevention’s independent advisory committee was set to consider and vote on whether to recommend the move. The committee is likely to give its approval, and the C.D.C. would follow with its own endorsement of the additional doses. “The F.D.A. is especially cognizant that immunocompromised people are particularly at risk for severe disease,” Dr. Janet Woodcock, the acting F.D.A. commissioner, said in a statement. “After a thorough review of the available data, the F.D.A. determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna Vaccines.” The authorization of the third doses kicks off what promises to be a busy next stretch for federal vaccine regulators — and a new phase of the nation’s inoculation drive. By the start of next month, the agency is expected to grant full approval to Pfizer-BioNTech’s vaccine. That will most likely prompt a wave of vaccination mandates from companies and organizations that waited to require vaccination until the F.D.A. fully cleared a vaccine. At the same time, government scientists and regulators are grappling with whether more Americans will need booster shots, a hotly debated move that many scientists argue is not yet supported by data. Other countries such as Israel and Germany have implemented booster policies. “Other individuals who are fully vaccinated are adequately protected and do not need an additional dose of Covid-19 vaccine at this time,” Dr. Woodcock said in her statement Thursday, adding that the agency was “actively engaged in a science-based, rigorous process with our federal partners to consider whether an additional dose may be needed in the future.” The United States is the latest country to begin offering third doses to those with weaker immune systems. France has offered additional vaccine doses to certain people with poor immune responses since April, and Germany and Hungary recently followed suit. About 3 percent of Americans have weakened immune systems for a variety of reasons, from a history of cancer to the use of certain medications such as steroids. The F.D.A.’s decision to limit the category of people with weakened immune systems who should receive the extra dose was expected. Many scientists argue that the immunocompromised population is too diverse to uniformly recommend additional shots of coronavirus vaccine. Some may be protected by the standard vaccine dosage, despite their conditions. Others may be poorly shielded by the vaccines, but unable to benefit from an additional shot. Studies suggest that patients such as organ transplant recipients are in between — often showing little immune response to the standard vaccine regimen, but benefiting from a third shot. One recent randomized, placebo-controlled study by Canadian researchers found that a third dose of the Moderna vaccine improved the immune response of people in that group. Noah Weiland is a reporter in the Washington bureau, covering health care. He was raised in East Lansing, Mich., and graduated from the University of Chicago. @noahweiland Sharon LaFraniere is an investigative reporter. She was part of a team that won a Pulitzer Prize in 2018 for national reporting on Donald Trump’s connections with Russia. @SharonLNYT

|
Scooped by
Juan Lama
|
Pfizer’s coronavirus vaccine may become slightly weaker over time, the company reported. But experts said that most people won’t need boosters anytime soon. Pfizer reported on Wednesday that the power of its two-dose Covid vaccine wanes slightly over time, but nonetheless offers lasting and robust protection against serious disease. The company suggested that a third shot could improve immunity, but whether boosters will be widely needed is far from settled, the subject of heated debate among scientists. So far, federal health officials have said boosters for the general population are unnecessary. And experts questioned whether vaccinated people should get more doses when so many people have yet to be immunized at all. “There’s not enough evidence right now to support that that is somehow the best use of resources,” said Natalie Dean, a biostatistician at Emory University in Atlanta. Still, the findings raise questions about how well the Pfizer vaccine will prevent infection in the months to come. And with coronavirus cases surging again in many states, the data may influence the Biden administration’s deliberations about delivering boostersfor older people. If third shots are cleared for the general population, the boosters would likely represent a multi-billion-dollar business for Pfizer. In a study posted online but not yet peer-reviewed or published in a scientific journal, Pfizer and BioNTech scientists reported that the vaccine had a sky-high efficacy rate of about 96 percent against symptomatic Covid-19 for the first two months following the second dose. But the figure declined by about 6 percent every two months after that, falling to 83.7 percent after about four to six months. Against severe disease, however, the vaccine’s efficacy held steady at about 97 percent. “This drop is very slight — I wouldn’t say it’s waning,” said Akiko Iwasaki, an immunologist at Yale University. She did not see in the new study any evidence that boosters should go into use for the general population. “These data don’t support a need for that right now,” she said. Your Coronavirus Tracker: We’ll send you the latest data for places you care about each day. The findings fit with what scientists have learned about how the immune system fends off viruses. Antibodies are the only defense to prevent an infection, but their levels typically drop in the months after vaccination or recovery from the disease. If the coronavirus takes hold, immune cells can swoop in to destroy infected cells and make new antibodies. That enduring defense produced by the vaccine may explain how the virus can sometimes breed in the nose — producing a cold or sore throat — but fail to reach the lung where it can cause serious disease. “Everything that’s engaged by the vaccine is able to fight off that spread that ultimately leads to severe disease,” Dr. Iwasaki said. “That’s probably not declining at all.” The study period ended before the rise of the Delta variant, the highly contagious version of the virus that now dominates in the United States and makes vaccines somewhat less effective against infection. The findings come from 42,000 volunteers in six countries who participated in a clinical trial that Pfizer and BioNTech began last July. Half of the volunteers got the vaccine, while the other half received a placebo. Both groups received two shots spaced three weeks apart. The researchers compared the number of people in each group who developed symptoms of Covid-19, which was then confirmed by a P.C.R. virus test. When the companies announced their first batch of results, the vaccine showed an efficacy against symptomatic Covid-19 of 95 percent. In other words, the risk of getting sick was reduced by 95 percent in the group that got the vaccine, compared with the group that got the placebo. That result — the first for any Covid-19 vaccine — brought an exhilarating dose of hope to the world in December when it was riding what had been the biggest wave of the pandemic. Since then, the Pfizer-BioNTech vaccine has made up the majority of shots that Americans have received, with more than 191 million doses given so far, according to the Centers for Disease Control. In the new study, the researchers followed the volunteers for six months after vaccination, up to March 13. Over the entire period, the researchers estimated, the vaccine’s efficacy was 91.5 percent against symptomatic Covid-19. (The study did not measure the rate of asymptomatic virus infections.) But within that period, efficacy did gradually drop. Between one week and two months after the second dose, the figure was 96.2 percent. In the period from two to four months following vaccination, efficacy fell to 90.1 percent. From four months after vaccination to the March cutoff, the figure was 83.7 percent. Those figures still describe a remarkably effective vaccine, however, and may not convince critics that booster shots are widely needed. The study comes on the heels of data from Israel suggesting that the Pfizer-BioNTech vaccine’s protection may be waning there. But experts have pushed back against a rush to approving a booster there. The data have too many sources of uncertainty, they say, to make a precise estimate of how much effectiveness has waned. For example, the Delta-driven outbreak hit parts of the country with high vaccination rates first and has been hitting other regions later. “Such an analysis is still highly uncertain,” said Doron Gazit, a physicist at Hebrew University who analyzes Covid-19 trends for the Israeli government. Earlier on Wednesday, Pfizer reported that a third dose of its vaccine significantly increases blood levels of antibodies against several versions of the virus, including the Delta variant. Results were similar for antibodies produced against the original virus and the Beta variant, which was first identified in South Africa. Pfizer and BioNTech expect to publish more definitive research in the coming weeks. The announcement was a preliminary snapshot of data contained in an earnings statement. And although antibody levels are an important measure of immunity, they are not the only metric. The body has other defenses that turn back infection. Pfizer also said in its statement that vaccines for children ages 5 through 11 years could be available as early as the end of September. The vaccine is already authorized in the United States for everyone ages 12 and up. Pfizer’s vaccine brought in $7.8 billion in revenue in the last three months, the company said, and is on track to generate more than $33.5 billion this year. The vaccine is poised to generate more sales in a single year than any previous medical product, and by a wide margin. Pfizer did not disclose its exact profits on the vaccine, but reiterated its previous estimate that its profit margins on the vaccine would be in the high 20 percent range. Even if the drugmaker’s profits fall on the lower end of that range, that would work out to about $3 billion in profit so far this year.

|
Scooped by
Juan Lama
|
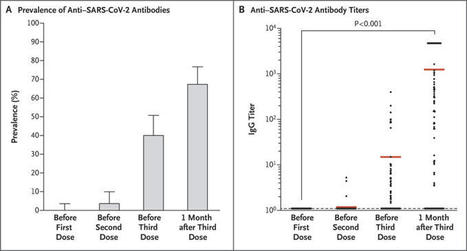
A weak immune response to two doses of vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been observed in recipients of solid-organ transplants.1,2 Severe cases of coronavirus disease 2019 (Covid-19) have also been reported in transplant recipients who had received two doses of vaccine.3 These reports prompted the French National Authority for Health to recommend the use of a third dose in immunosuppressed patients.4 Here, we report the humoral response in a group of 101 consecutive solid-organ transplant recipients (mean [±SD] age, 58±2 years; 69% were men) who were given three doses of the messenger RNA vaccine BNT162b2 (Pfizer–BioNTech). The group included 78 kidney-transplant recipients, 12 liver-transplant recipients, 8 lung-transplant or heart-transplant recipients, and 3 pancreas-transplant recipients. The first two doses were given 1 month apart, and the third dose was administered 61±1 days after the second dose. The time between transplantation and the initiation of vaccination was 97±8 months. Immunosuppression was due to the use of glucocorticoids (in 87% of patients), calcineurin inhibitors (in 79% of patients), mycophenolic acid (in 63% of patients), mammalian target of rapamycin inhibitors (in 30% of patients), and belatacept (in 12% of patients). The levels of antibodies to SARS-CoV-2 spike protein were assessed in all the patients with the use of the Wantai enzyme-linked immunosorbent assay (Beijing Wantai Biological Pharmacy Enterprise).5 Antibody titers are expressed as the ratio of the sample signal to a calibrator-assigned cutoff signal (the signal-to-cutoff ratio). According to French law, because this was an anonymous retrospective study, institutional review board approval was not required. The prevalence of anti–SARS-CoV-2 antibodies was 0% (95% confidence interval [CI], 0 to 4; 0 of 101 patients) before the first dose, 4% (95% CI, 1 to 10; 4 of 101 patients) before the second dose, 40% (95% CI, 31 to 51; 40 of 99 patients) before the third dose, and 68% (95% CI, 58 to 77; 67 of 99 patients) 4 weeks after the third dose (Figure 1). Among the 59 patients who had been seronegative before the third dose, 26 (44%) were seropositive at 4 weeks after the third dose (mean [±SD] signal-to-cutoff ratio, 690±293). All 40 patients who had been seropositive before the third dose were still seropositive 4 weeks later; their antibody titers increased from 36±12 before the third dose to 2676±350 1 month after the third dose (P<0.001). Patients who did not have an antibody response were older, had a higher degree of immunosuppression, and had a lower estimated glomerular filtration rate than patients who had an antibody response (see the Supplementary Appendix, available with the full text of this letter at NEJM.org). As of this writing, Covid-19 had not developed in any of the patients after they received the three vaccine doses. No serious adverse events were reported after the administration of the third dose, and no acute rejection episodes occurred. This study showed that administration of a third dose of the BNT162b2 vaccine to solid-organ transplant recipients significantly improved the immunogenicity of the vaccine, with no cases of Covid-19 reported in any of the patients. However, a large proportion of the patients remain at risk for Covid-19. Barrier measures should be maintained, and vaccination of the relatives of these patients should be encouraged. Published in NEJM (June 23, 2021): https://doi.org/10.1056/NEJMc2108861
|

|
Scooped by
Juan Lama
|
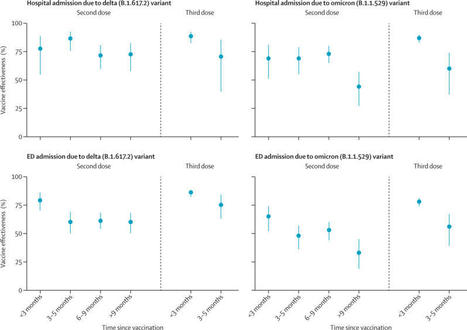
Background The duration of protection against the omicron (B.1.1.529) variant for current COVID-19 vaccines is not well characterised. Vaccine-specific estimates are especially needed. We aimed to evaluate the effectiveness and durability of two and three doses of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine against hospital and emergency department admissions due to the delta (B.1.617.2) and omicron variants. Methods In this case–control study with a test-negative design, we analysed electronic health records of members of Kaiser Permanente Southern California (KPSC), a large integrated health system in California, USA, from Dec 1, 2021, to Feb 6, 2022. Vaccine effectiveness was calculated in KPSC patients aged 18 years and older admitted to hospital or an emergency department (without a subsequent hospital admission) with a diagnosis of acute respiratory infection and tested for SARS-CoV-2 via PCR. Adjusted vaccine effectiveness was estimated with odds ratios from adjusted logistic regression models. This study is registered with ClinicalTrials.gov (NCT04848584). Findings Analyses were done for 11 123 hospital or emergency department admissions. In adjusted analyses, effectiveness of two doses of the BNT162b2 vaccine against the omicron variant was 41% (95% CI 21–55) against hospital admission and 31% (16–43) against emergency department admission at 9 months or longer after the second dose. After three doses, effectiveness of BNT162b2 against hospital admission due to the omicron variant was 85% (95% CI 80–89) at less than 3 months but fell to 55% (28–71) at 3 months or longer, although confidence intervals were wide for the latter estimate. Against emergency department admission, the effectiveness of three doses of BNT162b2 against the omicron variant was 77% (72–81) at less than 3 months but fell to 53% (36–66) at 3 months or longer. Trends in waning against SARS-CoV-2 outcomes due to the delta variant were generally similar, but with higher effectiveness estimates at each timepoint than those seen for the omicron variant. Interpretation Three doses of BNT162b2 conferred high protection against hospital and emergency department admission due to both the delta and omicron variants in the first 3 months after vaccination. However, 3 months after receipt of a third dose, waning was apparent against SARS-CoV-2 outcomes due to the omicron variant, including hospital admission. Additional doses of current, adapted, or novel COVD-19 vaccines might be needed to maintain high levels of protection against subsequent waves of SARS-CoV-2 caused by the omicron variant or future variants with similar escape potential. Published (April 22, 2022) in The Lancet Respiratory Medicine:

|
Scooped by
Juan Lama
|
Pfizer and BioNTech said three doses of their Covid-19 vaccine neutralize the Omicron coronavirus variant, according to the results of an initial laboratory study. The data showed a third dose of the vaccine increases neutralizing antibodies by 25-fold compared with two doses, strengthening the case for and need for booster shots. The preliminary data suggest three doses provide a similar level of antibodies as is observed after two doses against other variants that emerged before Omicron, the companies said. Two vaccine doses showed a 25-fold reduction in neutralizing antibodies against Omicron compared with other variants, which they said suggested two doses “may not be sufficient” protection. “Although two doses of the vaccine may still offer protection against severe disease caused by the Omicron strain, it’s clear from these preliminary data that protection is improved with a third dose of our vaccine,” Pfizer (ticker: PFE) CEO Albert Bourla said in a statement. The companies added that the development of an Omicron-specific vaccine was progressing and is expected to be available by March 2022, if such an adaptation ends up being needed. “The takeaways from Pfizer’s update underscore our belief that the durability of Pfizer’s vaccine sales for Covid-19 remain underappreciated by the Street,” Cantor Fitzgerald analysts said. They rate the stock as Overweight with a target price of $61, implying an 18% upside to Tuesday’s closing price. Another study, published late Tuesday, suggested that Pfizer’s Covid-19 vaccine offers partial protection against the Omicron variant. The small South African laboratory-based study suggested that the variant escapes antibody immunity induced by the Pfizer-BioNTech vaccine but that “considerable immunity” is retained. “It is likely that lesser vaccine-induced protection against infection and disease would be the result,” said Africa Health Research Institute’s executive director, Prof. Willem Hanekom. “Importantly most vaccinologists agree that the current vaccines will still protect against severe disease and death in the face of Omicron infection,” he added. The study was small, including blood samples from just 12 participants, but it is the first scientific data into the efficacy of vaccines against the Omicron variant. The loss of immune protection is “robust but not complete,” head of research Alex Sigal said, adding that a good booster shot would decrease the risk of infection, especially of more severe disease. The study concluded that previous infection followed by vaccination of booster was likely to provide protection. It has been a volatile couple of weeks for Covid-19 vaccine makers, as investors battle to assess the impact of the Omicron variant while waiting for answers to crucial questions. Moderna (MRNA), Pfizer and Novavax (NVAX) were among the sharpest fallers in U.S. premarket trading on Wednesday. That is after plunging Monday and rallying on Tuesday. But Pfizer stock reversed course following the study results and was trading 0.4% higher. Press release by Pfizer (Dec. 8, 2021) available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant

|
Scooped by
Juan Lama
|
Background: While Coronavirus disease 2019 (Covid-19) vaccines are highly effective, breakthrough infections are occurring. Booster vaccinations have recently received emergency use authorization (EUA) for certain populations but are restricted to homologous mRNA vaccines. We evaluated homologous and heterologous booster vaccination in persons who had received an EUA Covid-19 vaccine regimen. Methods: In this phase 1/2 open-label clinical trial conducted at ten U.S. sites, adults who received one of three EUA Covid-19 vaccines at least 12 weeks prior to enrollment and had no reported history of SARS-CoV-2 infection received a booster injection with one of three vaccines (Moderna mRNA-1273 100-mcg, Janssen Ad26.COV2.S 5x1010 virus particles, or Pfizer-BioNTech BNT162b2 30-mcg; nine combinations). The primary outcomes were safety, reactogenicity, and humoral immunogenicity on study days 15 and 29. Results: 458 individuals were enrolled: 154 received mRNA-1273, 150 received Ad26.CoV2.S, and 154 received BNT162b2 booster vaccines. Reactogenicity was similar to that reported for the primary series. Injection site pain, malaise, headache, and myalgia occurred in more than half the participants. Booster vaccines increased the neutralizing activity against a D614G pseudovirus (4.2-76-fold) and binding antibody titers (4.6-56-fold) for all combinations; homologous boost increased neutralizing antibody titers 4.2-20-fold whereas heterologous boost increased titers 6.2-76-fold. Day 15 neutralizing and binding antibody titers varied by 28.7-fold and 20.9-fold, respectively, across the nine prime-boost combinations. Conclusion: Homologous and heterologous booster vaccinations were well-tolerated and immunogenic in adults who completed a primary Covid-19 vaccine regimen at least 12 weeks earlier. Available as preprint in medRxiv (Oct. 13, 2021): https://doi.org/10.1101/2021.10.10.21264827

|
Scooped by
Juan Lama
|
In a highly unusual decision, the C.D.C. director, Rochelle Walensky, reversed a move by agency advisers to endorse additional doses of the Pfizer-BioNTech vaccine for older Americans but not for health care workers or teachers. The director of the Centers for Disease Control and Prevention on Friday overruled a recommendation by an agency advisory panel that had refused to endorse booster shots of the Pfizer-BioNTech Covid vaccine for frontline workers. It was a highly unusual move for the director, Dr. Rochelle Walensky, but aligned C.D.C. policy with the Food and Drug Administration’s endorsements over her own agency’s advisers. The C.D.C.’s Advisory Committee on Immunization Practices on Thursday recommended the boosters for a wide range of Americans, including tens of millions of older adults and younger people at high risk for the disease. But they excluded health care workers, teachers and others whose jobs put them at risk. That put their recommendations at odds with the F.D.A.’s authorization of booster shots for all adults with a high occupational risk. Dr. Walensky’s decision was a boost for President Biden’s campaign to give a broad swathe of Americans access to boosters. The White House had come under criticism for getting ahead of the regulatory process. The C.D.C.’s statement arrived well past midnight, a sign of the complicated and confusing decision-making surrounding the boosters. The C.D.C. advisers similarly spent two days debating who should get boosters and when, and could not agree on whether occupational risk should qualify as a criterion. “I am surprised that Dr. Walensky overturned one of the four A.C.I.P. votes today, and I believe others will be as well,” said Dr. Yvonne Maldonado, an infectious disease expert at Stanford and the American Academy of Pediatrics liaison to the committee. But the vote on boosters for occupational risk “was close,” Dr. Maldonado said, and agreed with Dr. Walensky’s decision. “This addresses not only waning immunity but those at high risk of exposure,” Dr. Maldonado added. Minutes before Dr. Walensky’s statement, Dr. Amanda Cohn, who oversaw the two-day meeting of the panel, tried to prepare the advisers for the director’s decision. “Dr. Walensky is reversing the decision to not recommend use of a booster dose in persons at high risk for occupational or institutional exposure,” Dr. Cohn wrote in the email. “I am hoping to share this news with you before you see it in the press.” Dr. Walensky’s decision to go against her own agency’s advisers came as a surprise to at least some of her staff members: The C.D.C. director’s endorsement of the advisory committee’s recommendations is typically just a formality. Hours before her statement, agency insiders predicted she would stick with the usual protocol because doing otherwise would undermine the process and upset the advisers as well as her own staff. But experts outside the C.D.C. said Dr. Walensky may have had no choice but to align herself with the F.D.A.’s decision. “There’s a complexity here, because Dr. Walensky was part of the White House announcement” on boosters, noted Dr. Ashish Jha, dean of the Brown University School of Public Health. Dr. Walensky said providing booster shots to health care workers and others who risk contracting the disease on the job would “best serve the nation’s public health needs.”

|
Scooped by
Juan Lama
|
It comes two days before an outside advisory committee of experts is scheduled to meet to recommend whether or not the agency should approve the company’s request. The Food and Drug Administration on Wednesday offered the first public look at Pfizer’s application for a booster coronavirus shot, two days before an outside advisory committee of experts is scheduled to meet to recommend whether or not the agency should approve the company’s request. It also comes amid significant disagreement about the need for boosters between career scientists at the agency and top Biden health officials, who have already started planning a broad booster campaign for this fall. In a 23-page document reviewing the company’s application, regulators examined safety and immune response data on roughly 300 adults who received a booster shot of Pfizer-BioNTech’s vaccine six months after their second dose, finding an increased immune response in study participants, even as they said that coronavirus vaccines were holding up powerfully against severe forms of Covid-19. There were no serious safety concerns associated with the booster injection, the regulators reported. Pfizer said in a separate filing that one month after a third injection, levels of neutralizing antibodies against the Delta variant in a subgroup of trial volunteers were between five and seven times higher, roughly, than they were a month after the second dose. The company also reiterated its findings that the effectiveness of its vaccine against symptomatic disease fell from about 96 percent to about 84 percent by six months after the second shot, although it held steady against severe disease. Pfizer argued in its filing that ebbing of the vaccine’s potency was the dominant reason for breakthrough infections among vaccinated people in Israel, which has relied almost exclusively on the Pfizer vaccine and has vaccinated its population faster than the United States. But the F.D.A. regulators wrote that while waning immunity is one potential factor in breakthrough infections, other variables, including the Delta variant, may also have contributed to the cases. In an interview, Pfizer officials acknowledged that the company’s booster study was quite small. But they said that the data they have delivered meets the F.D.A.’s criteria for justifying third shots for people 16 and up. Pfizer has another, much bigger booster study underway, with results expected this fall. The F.D.A.’s analysis noted that Pfizer provided data on immune response against the Delta variant, by far the dominant variant in the U.S., in only two dozen people. Understanding the effectiveness of boosters against variants would likely be critical to the F.D.A.’s review, the document suggested. “Available data should support the effectiveness of the booster dose, particularly against currently circulating” variants, regulators wrote. The analysis also suggested that regulators are cautiously weighing studies from Israel, which top Biden administration officials have said were key to their decision to recommend starting a booster campaign this month. Israel is already providing booster shots to most of its population. “While observational studies can enable understanding of real-world effectiveness, there are known and unknown biases that can affect their reliability,” the regulators wrote. Studies in the United States “may most accurately represent vaccine effectiveness in the U.S. population,” they added. FDA Publication available (Sept. 17, 2021): https://www.fda.gov/media/152176/download Noah Weiland covers the coronavirus pandemic as a health reporter in the Washington bureau of The New York Times. He was part of a team that won a Pulitzer Prize in 2021 for its coverage of Covid-19. He grew up in East Lansing, Mich., and graduated from the University of Chicago. @noahweiland Sharon LaFraniere is an investigative reporter. She was part of a team that won a Pulitzer Prize in 2018 for national reporting on Donald Trump’s connections with Russia. @SharonLNYT

|
Scooped by
Juan Lama
|
Israel's Prime Minister has announced a program to roll out a third dose of the coronavirus vaccine to people over the age of 60, becoming one of the first countries in the world to make such a move. People over 60 will need to show they received their second dose of the vaccine at least five months ago. Thursday's announcement follows a strong recommendation from the government-appointed team of experts on the pandemic to offer older adults a third dose. The experts' advice, which came overnight on Wednesday, was based on data suggesting significant waning immunity from infection over time. Some of the data considered by the health ministry comes from research on the "justification, safety and efficacy" of a third dose of the Pfizer-BioNTech vaccine for hemodialysis patients that was posted as a pre-print paper earlier this month. The research found that about two-thirds of hemodialysis patients (people who require the procedure to remove waste products and excess fluid from the blood when the kidneys stop working properly) who had a suboptimal immune response after a second dose of the vaccine developed "optimal" antibodies and T cells after a third dose. From Tuesday to Thursday, the number of new cases in Israel has topped two thousand each day -- levels that have not been seen in the country for four and half months. Back in May and June, the number of new daily cases was down to single figures on some days. The number of severe cases currently stands at 151, with the R rate -- the average number of people infected by someone with the virus -- fairly steady for weeks, at 1.3 and 1.4. Israel's highly successful vaccination program first began in December, with then-Prime Minister Benjamin Netanyahu the first to receive a dose on live television. The country's vaccination program has won plaudits for its fast rate of making the vaccine available to the entire adult population, and more recently children aged 12 and over. Data from Israel might help inform other countries' decisions to offer a booster shot, including the United States. On Thursday, US Surgeon General Dr. Vivek Murthy told CNN that it's "very possible" that a decision on boosters will be made by the end of summer or early fall. "It could take a bit longer. It could come sooner," Murthy added, saying that "it depends how quickly we see a signal in the data in these cohorts of individuals we're following." Murthy said data from other countries, including Israel and the US will factor into the decision.

|
Scooped by
Juan Lama
|
Antibodies naturally wane over time, so studies are underway to tell if and when booster shots might be needed. Pfizer is about to seek U.S. authorization for a third dose of its Covid-19 vaccine, saying Thursday that another shot within 12 months could dramatically boost immunity and maybe help ward off the latest worrisome coronavirus mutant. Research from multiple countries shows the Pfizer shot and other widely used Covid-19 vaccines offer strong protection against the highly contagious Delta variant, which is spreading rapidly around the world and now accounts for most new U.S. infections. Two doses of most vaccines are critical to develop high levels of virus-fighting antibodies against all versions of the coronavirus, not just the Delta variant — and most of the world still is desperate to get those initial protective doses as the pandemic continues to rage. But antibodies naturally wane over time, so studies also are underway to tell if and when boosters might be needed. On Thursday, Pfizer’s Mikael Dolsten told the Associated Press that early data from the company’s booster study suggests people’s antibody levels jump five- to 10-fold after a third dose, compared to their second dose months earlier. In August, Pfizer plans to ask the Food and Drug Administration for emergency authorization of a third dose, he said. Why might that matter for fighting the Delta variant? Dolsten pointed to data from Britain and Israel showing the Pfizer vaccine “neutralizes the Delta variant very well.” The assumption, he said, is that when antibodies drop low enough, the Delta virus eventually could cause a mild infection before the immune system kicks back in. But FDA authorization would be just a first step — it wouldn’t automatically mean Americans get offered boosters, cautioned William Schaffner, a vaccine expert at Vanderbilt University Medical Center. Public health authorities would have to decide if they’re really needed, especially since millions of people have no protection. “The vaccines were designed to keep us out of the hospital” and continue to do so despite the more contagious Delta variant, he said. Giving another dose would be “a huge effort while we are at the moment striving to get people the first dose.” Hours after Pfizer’s announcement, U.S. health officials issued a statement saying fully vaccinated Americans don’t need a booster yet. U.S. health agencies “are engaged in a science-based, rigorous process to consider whether or when a booster might be necessary,” the FDA and Centers for Disease Control and Prevention said in a joint statement. That work will include data from the drug companies, “but does not rely on those data exclusively,” and any decision on booster shots would happen only when “the science demonstrates that they are needed,” the agencies said. Currently only about 48% of the U.S. population is fully vaccinated — and some parts of the country have far lower immunization rates, places where the Delta variant is surging. On Thursday, Rochelle Walensky, the CDC director, said that’s leading to “two truths” — highly immunized swaths of America are getting back to normal while hospitalizations are rising in other places. “This rapid rise is troubling,” she said: A few weeks ago the Delta variant accounted for just over a quarter of new U.S. cases, but it now accounts for just over 50% — and in some places, such as parts of the Midwest, as much as 80%. Also Thursday, researchers from France’s Pasteur Institute reported new evidence that full vaccination is critical. In laboratory tests, blood from several dozen people given their first dose of the Pfizer or AstraZeneca vaccines “barely inhibited” the Delta variant, the team reported in the journal Nature. But weeks after getting their second dose, nearly all had what researchers deemed an immune boost strong enough to neutralize the Delta variant — even if it was a little less potent than against earlier versions of the virus. The French researchers also tested unvaccinated people who had survived a bout of the coronavirus, and found their antibodies were four-fold less potent against the new mutant. But a single vaccine dose dramatically boosted their antibody levels — sparking cross-protection against the Delta variant and two other mutants, the study found. That supports public health recommendations that Covid-19 survivors get vaccinated rather than relying on natural immunity. The lab experiments add to real-world data that the Delta variant’s mutations aren’t evading the vaccines most widely used in Western countries, but underscore that it’s crucial to get more of the world immunized before the virus evolves even more. Researchers in Britain found two doses of the Pfizer vaccine, for example, are 96% protective against hospitalization with the Delta variant and 88% effective against symptomatic infection. That finding was echoed last weekend by Canadian researchers, while a report from Israel suggested protection against mild Delta infection may have dipped lower, to 64%. Whether the fully vaccinated still need to wear masks in places where the Delta variant is surging is a growing question. In the U.S., the CDC maintains that fully vaccinated people don’t need to. Even before the Delta variant came along, the vaccines weren’t perfect, but the best evidence suggests that if vaccinated people nonetheless get the coronavirus, they’ll have much milder cases. “Let me emphasize, if you were vaccinated, you have a very high degree of protection,” Anthony Fauci, the U.S. government’s top infectious disease expert, said Thursday. In the U.S., case rates have been rising for weeks and the rate of hospitalizations has started to tick up, rising 7% from the previous seven-day average, Walensky told reporters Thursday. However, deaths remain down on average, which some experts believe is at least partly due to high vaccination rates in people 65 and older — who are among the most susceptible to severe disease. Associated Press writer Mike Stobbe contributed to this story. The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
|



 Your new post is loading...
Your new post is loading...