FDA said it will ease up vetting general health and wellness apps, but it will scrutinize clinical applications and devices. Does this mean the FTC will step up?
The U.S. Food and Drug Administration has issued final guidance on “low-risk” digital health apps and devices for general health management 18 months after it came out with draft guidance.
The document offers information on the kinds of apps and devices for which it will and won’t take action. Apps promoting or maintaining a healthy weight or to assist with weight loss goals and healthy eating are OK. The guidance says that companies can make claims that their apps and devices can help with healthy lifestyle choices to reduce the risk of chronic conditions such as Type 2 diabetes, high blood pressure and heart disease or improve their management. But those lifestyle choices have to be advocated by the likes of the American Heart Association or American Association of Clinical Endocrinologist or peer-reviewed medical journals.
So what are some examples of what’s not OK? Claims that a product will treat or diagnose obesity, an eating disorder, such as bullimia or anorexia, or an anxiety disorder. Digital health entrepreneurs are also encouraged to ask themselves the following questions:
Is the product invasive?
Is the product implanted?
Does the product involve an intervention or technology that may pose a risk to
the safety of users and other persons if specific regulatory controls are not applied, such as risks from lasers or radiation exposure?
If the answer is yes to any of the above, they need to assume their products are considered clinical applications, will be scrutinized and should act accordingly.
My takeaway from the guidance is twofold. It’s a question of resources. Although there are thousands of general wellness apps, more and more medical device and pharma companies are developing digital health devices and apps of their own. Second, the Federal Trade Commission has shown it is willing to take action against companies that it deems to be making false health claims about their apps and devices.
Via rob halkes, Pharma Guy



 Your new post is loading...
Your new post is loading...

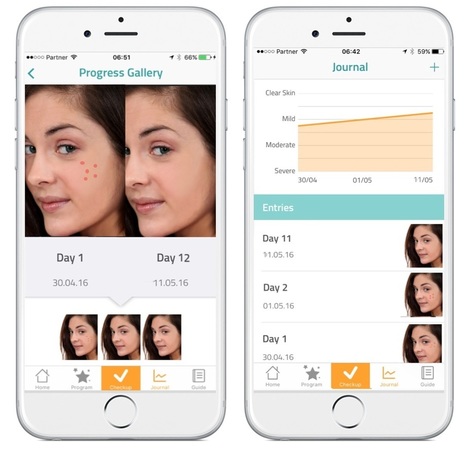






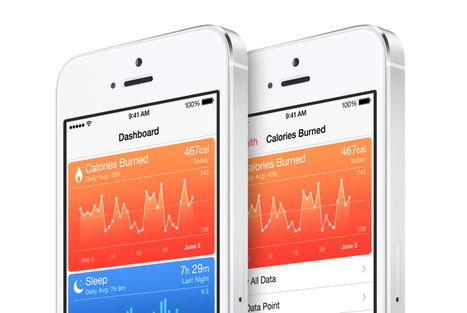









Health apps should do what they promise! At the moment they need to take a diagnostic feature and use personal physics to arrive at advice or conclusions about the health status of the person who uses the app, they are considered not to be 'just' an "app" but a medical device. At that condition they need to adhere to and be certified by several criteria attached to 'medical devices". Developers should know about this, which the more professional ones will. Rightly so!
PatientView has developed a website MyHealthApps that presents an inventory of the better Health Apps.
Also read “FDA Won't Regulate ‘Low-Risk’ mHealth Apps as Medical Devices. But Battle Looms Over Defining ‘Low Risk’"; http://sco.lt/5kkDyr